2025-11-24 スウェーデン王立工科大学(KTH)
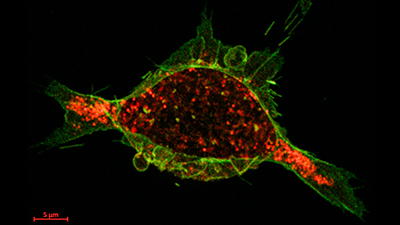
The calcium-regulated protein drug (green) and tumor cell receptors (red) have bonded and internalization is underway, 40 seconds after administration.
<関連情報>
- https://www.kth.se/en/om/nyheter/centrala-nyheter/calcium-sensitive-switch-designed-to-boost-efficacy-of-cancer-drugs-1.1442027
- https://www.pnas.org/doi/10.1073/pnas.2509081122
効率的な内部移行とリソソーム毒素送達のための設計されたカルシウム調節親和性タンパク質 Engineered calcium-regulated affinity protein for efficient internalization and lysosomal toxin delivery
Malin Jönsson, Marit Möller, Leon Schierholz, +6 , and Sophia Hober
Proceedings of the National Academy of Sciences Published:November 25, 2025
DOI:https://doi.org/10.1073/pnas.2509081122
Significance
This study introduces a calcium-regulated protein domain (CaRAEGFR) engineered for efficient internalization and targeted toxin delivery in cancer cells. By exploiting calcium gradients to control binding affinity, this approach enables precise, receptor-independent drug delivery to lysosomes, achieving potent cytotoxicity (IC50 = 0.8 nM) with the potential to avoid receptor downregulation. This proof-of-concept marks the use of calcium-regulated affinity (CaRA) in a small protein scaffold, offering a groundbreaking strategy to enhance specificity, efficacy, and safety in targeted cancer therapy.
Abstract
The emerging strategy of protein–drug conjugates (PDCs) for targeted cancer therapy holds great potential to improve treatment efficacy by specifically targeting cancer biomarkers and delivering toxic payloads directly to tumor cells, minimizing off-target toxicity. The success of this approach depends on the internalization and retention of the payload in target cells. This study introduces a method using a small protein domain engineered for conditional target affinity, enabling lysosomal trafficking independent of the biological fate of the receptor. Specifically, we describe the development of an EGF receptor binder, CaRAEGFR, with calcium-regulated affinity (CaRA), meaning the target binding strength is tailored by the available calcium concentration. This allows for endosomal dissociation, as calcium levels are lower in endosomes than in the bloodstream. Affinity measurements and structural modeling reveal the molecular basis of the calcium modulated affinity. Live cell imaging demonstrates efficient internalization and lysosomal trafficking of the calcium-dependent domain, while the EGF receptor is recycled to the membrane. When used as a drug carrier, CaRAEGFR effectively delivers the toxin to the lysosomes, resulting in potent cytotoxicity with an IC50 of 0.8 nM in EGFR-expressing cancer cells