2025-11-25 東京大学
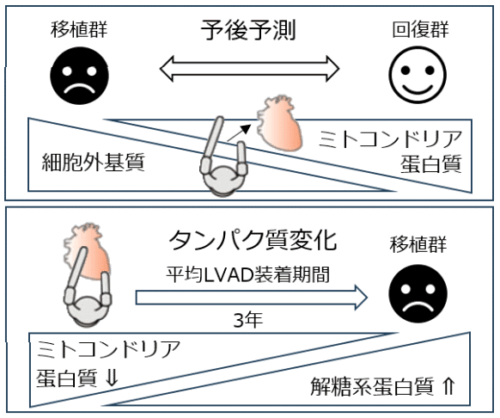
本研究の概要図(心臓プロテオーム解析により心不全予後予測タンパク質が同定)
<関連情報>
- https://www.h.u-tokyo.ac.jp/press/20251125.html
- https://www.h.u-tokyo.ac.jp/press/__icsFiles/afieldfile/2025/11/25/release_20251125.pdf
- https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.124.073093
機械的負荷軽減後の心臓回復に関わるプロテオームシグネチャー
Proteomic Signatures Involved in Cardiac Recovery After Mechanical Unloading
Yukako Shintani-Domoto, MD, PhD, Koji L. Ode, PhD, Seitaro Nomura, MD, PhD, Yoshiki Nagashima, Osamu Kinoshita, MD, PhD, Manami Katoh, MD, PhD, Takanobu Yamada, MD, PhD, …, and Masashi Fukayama, MD, PhD
Circulation Published: 24 November 2025
DOI:https://doi.org/10.1161/CIRCULATIONAHA.124.073093
Heart failure is a leading cause of death worldwide, and patients with severe heart failure require left ventricular assist device (LVAD) support.1 LVAD is usually implanted as a bridge to transplantation (BTT) or destination but can be removed if the patient’s cardiac function recovers. However, the molecular predictors for cardiac prognosis after LVAD implantation remain elusive.
We performed liquid chromatography–mass spectrometry on myocardial samples from heart transplant recipients, including 14 BTT cases and 10 bridge to recovery (BTR) cases, excluding patients with myocarditis. This study was approved by the institutional review board of the University of Tokyo (No. 10162, G10032). The data that support the findings of this study are available from the first authors upon reasonable request. LVAD implantation duration averaged 849 days (range, 68–1,405) in BTT and 202.8 days (range, 77–428) in BTR (Figure [A]). Myocardial tissues were processed, and formalin-fixed and paraffin-embedded specimens were analyzed. Samples from BTT were collected at both LVAD implantation (pre-LVAD) and heart transplantation (post-LVAD), whereas BTR samples were collected only at LVAD implantation. The samples were analyzed by data-dependent tandem mass spectrometry with a mass spectrometer (Q-Exactive Mass Spectrometer, Thermo Fisher Scientific). Mass spectrometry data were analyzed and quantified using Proteome Discoverer version 1.4 (Thermo Fisher Scientific) with the Swiss-Prot section of the UniProtKB human database (as of May 28, 2014). Only proteins that were consistently quantified in at least 11 of 14 BTT patients and 8 out of 10 BTR patients were included in the statistical analysis.