2025-12-08 奈良先端科学技術大学院大学,科学技術振興機構
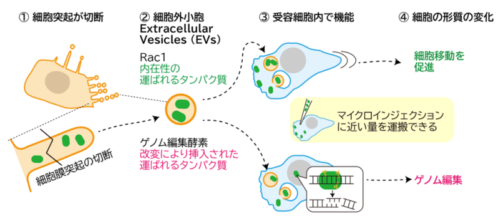
図 2 今回の研究:具体的な送達タンパク質の定量と改変(ゲノム編集酵素の挿入)
<関連情報>
- https://www.jst.go.jp/pr/announce/20251208/index.html
- https://www.jst.go.jp/pr/announce/20251208/pdf/20251208.pdf
- https://www.nature.com/articles/s41467-025-66351-1
突起由来細胞外小胞を介したタンパク質送達による効率的な細胞形質転換 Efficient cellular transformation via protein delivery through the protrusion-derived extracellular vesicles
Toshifumi Fujioka,Tamako Nishimura,Hiroki Kawana,Koichiro M. Hirosawa,Renta Yamakawa,Hani Sapili,Kayoko Oono-Yakura,Ryoya Nakagawa,Takanari Inoue,Osamu Nureki,Kenichi G. N. Suzuki & Shiro Suetsugu
Nature Communications Published:08 December 2025
DOI:https://doi.org/10.1038/s41467-025-66351-1
Abstract
Extracellular vesicles (EVs) mediate the transfer of intracellular proteins from producer to recipient cells. EVs originate either from plasma membrane protrusions or endosomes, with endosome-derived EVs being extensively studied and engineered. However, the efficiency and functionality of protein transfer via both types of EVs remain poorly understood. Here, we demonstrate that natural EVs derived from cell protrusions dependent on the I-BAR protein MIM, rather than from endosomes, deliver the functional small GTPase Rac1 protein at levels similar to microinjection. Rac1-containing EVs are internalized via endocytosis, trafficked through endosomal compartments, and subsequently released into the cytosol, where they enhance cell motility. To evaluate broader applicability, the genome-editing protein Cas12f is packaged into protrusion-derived EVs by MIM and endosome-derived EVs by endosomal tetraspanin CD63. Notably, protrusion-derived EVs deliver Cas12f with significantly higher efficiency than endosome-derived EVs, highlighting their superior capability for functional protein transfer. Our findings establish the protrusion-derived EVs as a powerful platform for the efficient and bioactive delivery of both native and engineered proteins, expanding the EV-based therapeutic strategies.