2025-12-17 マックス・プランク研究所

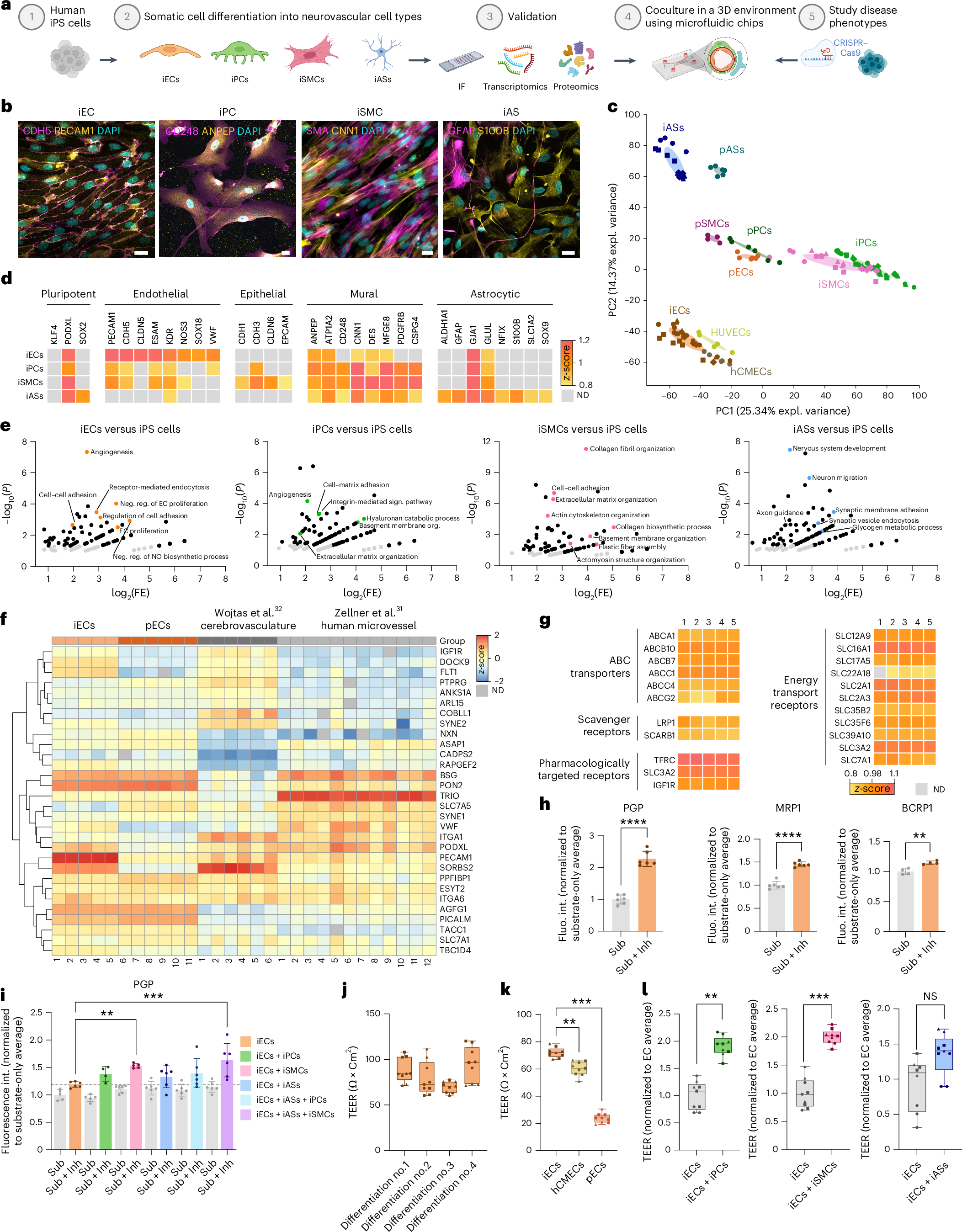
Human periportal assembloid, showcasing the three key cell types of the liver: portal fibroblasts (magenta), cholangiocytes (green), and hepatocyte nuclei (blue). All cell borders are delineated in white. © Lei Yuan, Sagarika Dawka, Yohan Kim, Anke Liebert et al. / Nature (2025) / MPI-CBG
<関連情報>
- https://www.mpg.de/25903861/1216-mozg-patient-specific-human-liver-model-to-understand-disease-mechanisms-151300-x
- https://www.nature.com/articles/s41586-025-09884-1
ヒトアセンブロイドはin vitroで門脈周囲肝組織を再現する Human assembloids recapitulate periportal liver tissue in vitro
Lei Yuan,Sagarika Dawka,Yohan Kim,Anke Liebert,Fabian Rost,Robert Arnes-Benito,Franziska Baenke,Christina Götz,David Long Hin Tsang,Andrea Schuhmann,Anna Shevchenko,Roberta Rezende de Castro,Seunghee Kim,Aleksandra Sljukic,Anna M. Dowbaj,Andrej Shevchenko,Daniel Seehofer,Dongho Choi,Georg Damm,Daniel E. Stange & Meritxell Huch
Nature Published:17 December 2025
DOI:https://doi.org/10.1038/s41586-025-09884-1
Abstract
The development of complex multicellular human in vitro systems holds great promise for modelling disease and advancing drug discovery and tissue engineering1. In the liver, despite the identification of key signalling pathways involved in hepatic regeneration2,3, in vitro expansion of human hepatocytes directly from fresh patient tissue has not yet been achieved, limiting the possibility of modelling liver composite structures in vitro. Here we first developed human hepatocyte organoids (h-HepOrgs) from 28 different patients. Patient-derived hepatocyte organoids sustained long-term expansion of hepatocytes in vitro and maintained patient-specific gene expression and bile canaliculus features and function of the in vivo tissue. After transplantation, expanded h-HepOrgs rescued the phenotype of a mouse model of liver disease. By combining h-HepOrgs with portal mesenchyme and our previously published cholangiocyte organoids4,5,6, we generated patient-specific periportal liver assembloids that retain the histological arrangement, gene expression and cell interactions of periportal liver tissue, with cholangiocytes and mesenchyme embedded in the hepatocyte parenchyma. We leveraged this platform to model aspects of biliary fibrosis. Our human periportal liver assembloid system represents a novel in vitro platform to investigate human liver pathophysiology, accelerate drug development, enable early diagnosis and advance personalized medicine.