2025-12-17 コロンビア大学
<関連情報>
- https://magazine.columbia.edu/article/scientist-teaching-cancer-self-destruct
- https://www.sciexplor.com/articles/fos.2025.0003
- https://www.cell.com/fulltext/S0092-8674(12)00520-X
持続癌細胞における脂質組成の変化がフェロプトーシス感受性の増強を引き起こす Lipidomic changes in persister cancer cells drive enhanced ferroptosis sensitivity
Eduard Reznik,Fereshteh Zandkarimi,Joleen M. Csuka,Qiulin Zhu,Jenny Jin,Taruna Vani Neelakantan,Jiewen Zheng,Vasiliki Polychronidou,Mark Fongheiser,Ashley Brown,Baiyu Qiu,Megan Rodriguez,Lela DeVine,Prem S Subramaniam,Wei Gu,Andrea Califano,Brent R. Stockwell
Ferroptosis and Oxidative Stress Published: November 10, 2025
DOI:https://doi.org/10.70401/fos.2025.0003
Abstract
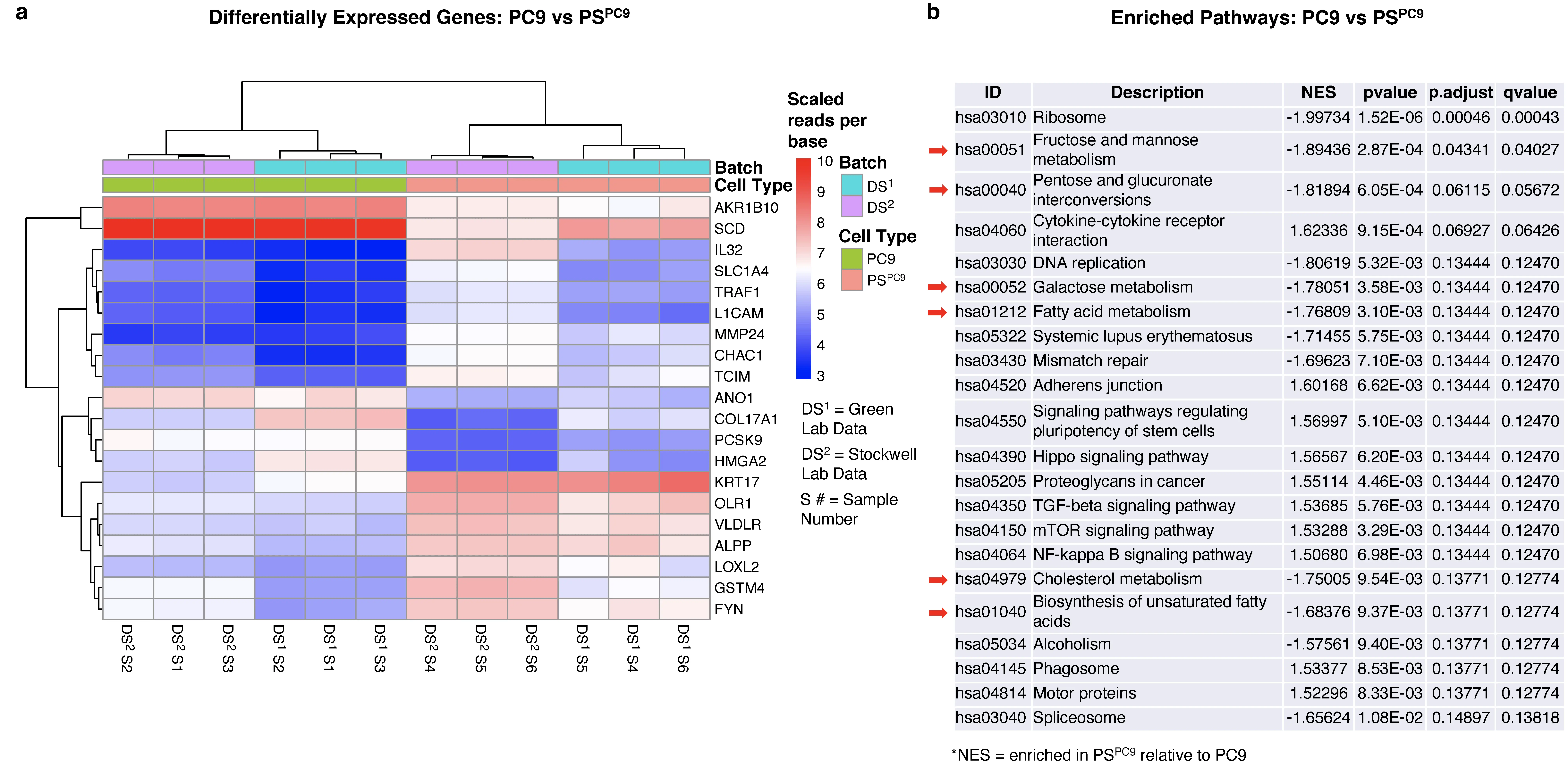
Aims: Unique in the broader category of drug-resistant cells, persister cancer cells (PSs) acquire their tolerance to compounds through reversible, chromatin-mediated changes, allowing them to ‘persist’ in the face of cancer therapeutic agents. PSs are implicated in minimal residual disease from which cancer relapse occurs, and given their established sensitivity to ferroptosis, PSs present a critical point through which identification and targeting of drug-resistant cancers may be possible. Ferroptosis sensitivity in drug-resistant cancers may be caused by the attainment of the persister state, or it may merely be correlative with this state and due instead to extended inhibition of oncogenic signaling or the induction of chemotherapy stress. Nonetheless, ferroptosis sensitivity has emerged as a common phenotype across multiple PS and drug-resistant cancer cell types. Identifying biomarkers for and drivers of ferroptosis sensitivity in drug-resistant and PS cells is therefore a high priority.
Methods: We derived PS cells from the lung carcinoma cell line PC9 (PSPC9), performed transcriptomic analysis, and subsequently lipidomics on the PC9/PSPC9 system. Additionally, we reverted PSPC9 cells to the ferroptosis-resistant parental state (PC9PS -> PC9) and assessed the resulting lipid changes. We generated two additional PS-like cell models: PS-like prostate carcinoma (PSLNCaP) from LNCaP cells and PS-like fibrosarcoma (PSHT1080) from HT1080 cells, with lipidomics analysis. Finally, we performed a mitochondrial elimination assay and assessed its effect on ferroptosis sensitivity.
Results: We observed enrichment of lipid and sugar metabolism gene expression in PSPC9; lipidomics revealed enrichment within PSPC9 for ferroptosis-driving diPUFA phospholipids (diPUFA-PL), as well as polyunsaturated free fatty acids (PUFA FFAs). Upon PSPC9 reversion to the ferroptosis-resistant parental state (PC9PS -> PC9), this lipid signature reverted. The LNCaP and HT1080 PS-like models individually showed features consistent with PS, including an increased labile-iron pool, reversibility, and enhanced ferroptosis sensitivity, and had lipid features consistent with those in PSPC9. Finally, mitochondrial elimination partially abrogated ferroptosis sensitivity and altered the PS lipid profile.
Conclusion: In summary, lipidomic changes dependent on the presence of mitochondria are key to the ferroptosis sensitivity of drug-tolerant persister cancer cells.
フェロプトーシス:鉄依存性の非アポトーシス性細胞死 Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death
Scott J. Dixon ∙ Kathryn M. Lemberg ∙ Michael R. Lamprecht ∙ … ∙ Wan Seok Yang ∙ Barclay Morrison, III ∙ Brent R. Stockwell
Cell Published:May 25, 2012
DOI:https://doi.org/10.1016/j.cell.2012.03.042
Summary
Nonapoptotic forms of cell death may facilitate the selective elimination of some tumor cells or be activated in specific pathological states. The oncogenic RAS-selective lethal small molecule erastin triggers a unique iron-dependent form of nonapoptotic cell death that we term ferroptosis. Ferroptosis is dependent upon intracellular iron, but not other metals, and is morphologically, biochemically, and genetically distinct from apoptosis, necrosis, and autophagy. We identify the small molecule ferrostatin-1 as a potent inhibitor of ferroptosis in cancer cells and glutamate-induced cell death in organotypic rat brain slices, suggesting similarities between these two processes. Indeed, erastin, like glutamate, inhibits cystine uptake by the cystine/glutamate antiporter (system x-c), creating a void in the antioxidant defenses of the cell and ultimately leading to iron-dependent, oxidative death. Thus, activation of ferroptosis results in the nonapoptotic destruction of certain cancer cells, whereas inhibition of this process may protect organisms from neurodegeneration.