2025-12-24 生理学研究所
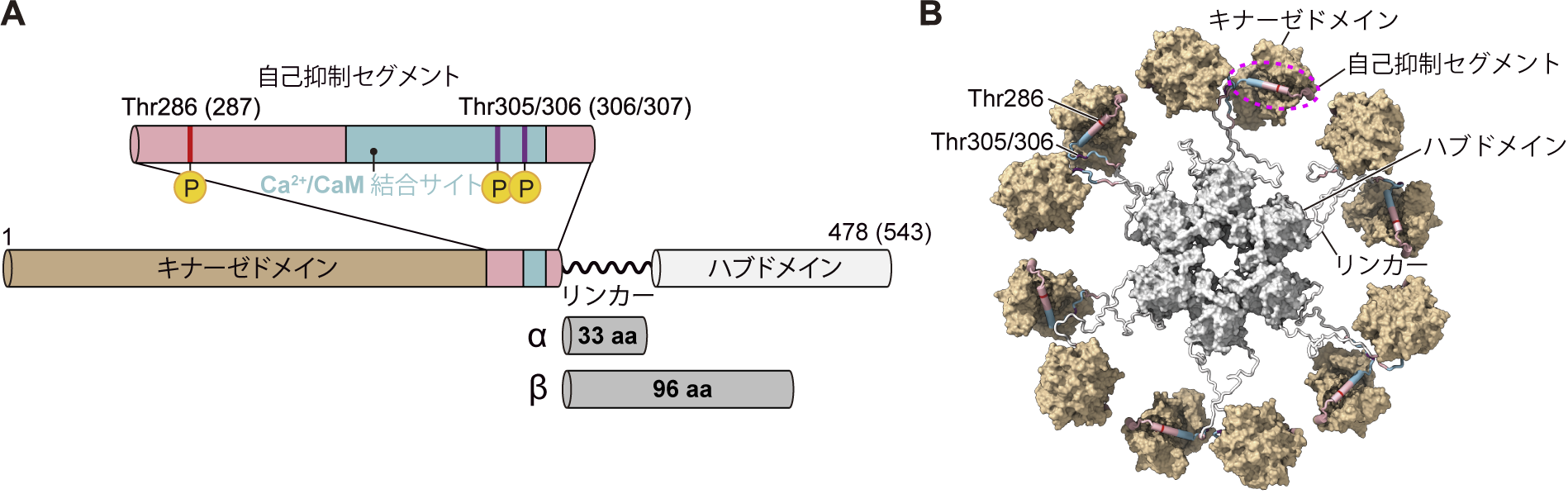
図 1:CaMKIIのドメイン構成。(A)括弧内の数字はβ型のアミノ酸残基を示す。α型とβ型の主な違いはリンカーのアミノ酸残基数である。電子顕微鏡画像に基づいて予想されたCaMKIIαの12量体構造モデル(B)。
<関連情報>
- https://www.nips.ac.jp/release/2025/12/camkii.html
- https://www.nature.com/articles/s41467-025-66527-9
高速AFMを用いたCaMKIIα/βが混合したヘテロ12量体のナノ動態イメージング Structural dynamics of mixed-subunit CaMKIIα/β heterododecamers filmed by high-speed AFM
Keisuke Matsushima,Takashi Sumikama,Taisei Suzuki,Mizuho Ito,Yutaro Nagasawa,Ayumi Sumino,Holger Flechsig,Tomoki Ogoshi,Kenichi Umeda,Noriyuki Kodera,Hideji Murakoshi & Mikihiro Shibata
Nature Communications Published:24 December 2025
DOI:https://doi.org/10.1038/s41467-025-66527-9
Abstract
CaMKII predominantly assembles into a 12-meric ring assembly, primarily consisting of α and β isoforms in the brain. Previous biochemical studies reported varying ratios of these CaMKII variants across different brain regions and developmental stages. However, direct evidence for the formation of CaMKIIα/β heterooligomers within a 12-meric ring assembly has been lacking at the single-molecule level. Here, we employ high-speed atomic force microscopy to visualize the conformational dynamics of forebrain-mimicked CaMKIIα/β at a 3:1 ratio. Our findings reveal that the α and β subunits are intermixed within the 12-meric ring assembly, with a probability exceeding 83% that β subunits are positioned adjacently. Furthermore, in the activated state, CaMKIIα/β heterooligomers form a stable kinase domain complex via interactions between adjacent CaMKIIβ subunits, resulting in a long-lasting structure with an exposed target binding site. Collectively, our observations provide insights into the structural role of CaMKIIβ subunits within the CaMKIIα/β heterododecamer.