2026-01-16 千葉大学医学部附属病院,理化学研究所
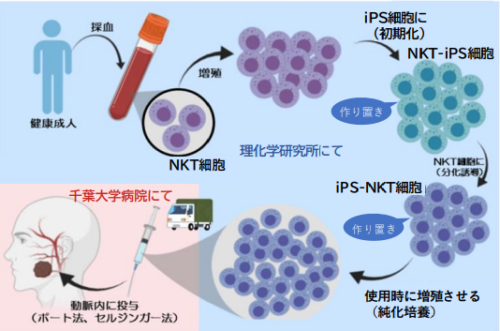
図1【iPS-NKT細胞の作製から投与までの流れ】
凍結保存したiPS-NKT細胞を投与タイミングに合わせて数を増やして使用
<関連情報>
- https://www.ho.chiba-u.ac.jp/hosp/assets/dl/about/topics_260119.pdf
- https://www.nature.com/articles/s41467-025-66801-w
再発性頭頸部癌における同種iPSC由来iNKT細胞:第1相試験 Allogeneic iPSC-derived iNKT cells in recurrent head and neck cancer: a phase 1 trial
Tomohisa Iinuma,Tomoya Kurokawa,Takahiro Aoki,Atsushi Onodera,Tominaga Fukazawa,Daisuke Yamada,Genta Kitahara,Momoko Okoshi,Munechika Yamaguchi,Hiroko Okura,Satoko Sasaki,Yoshie Sasako,Sachiko Kira,Jafar Sharif,Yukio Tsuchiyama,Midori Kobayashi,Norihiko Kobayashi,Takuro Horikoshi,Yosuke Inaba,Hideki Hanaoka,Yoshitaka Okamoto,Toyoyuki Hanazawa,Haruhiko Koseki & Shinichiro Motohashi
Nature Communications Published:26 November 2025
DOI:https://doi.org/10.1038/s41467-025-66801-w
Abstract
Invariant Natural killer T (iNKT) cells exhibit cytotoxic activity and immunomodulatory functions and have gained interest in cancer immunotherapy. We conducted a phase 1, first-in human clinical trial to evaluate the safety and efficacy of clinical-grade allogeneic iNKT cells generated from induced pluripotent stem cells (iPSC-iNKT cells) in patients with recurrent head and neck cancer (jRCT2033200116). The primary endpoint was the incidence of dose-limiting toxicity (DLT). The secondary endpoints were to assess safety and efficacy, as well as to evaluate immunological dynamics. iPSC-iNKT cells were administered intra-arterially to 10 patients. One subject developed grade 3 skin rash at the second dose, identified as DLT. No other severe adverse events were observed in any patients. Tumor progression was suppressed in two patients, in whom clonal expansion of memory- and effector-phenotype CD8+ T cells was observed, along with activation of the IFN-γ signaling pathway. Here, we show that iPSC-iNKT cells are safe and possess therapeutic potential as an immunotherapy for solid tumors.