2026-01-23 中国科学院(CAS)
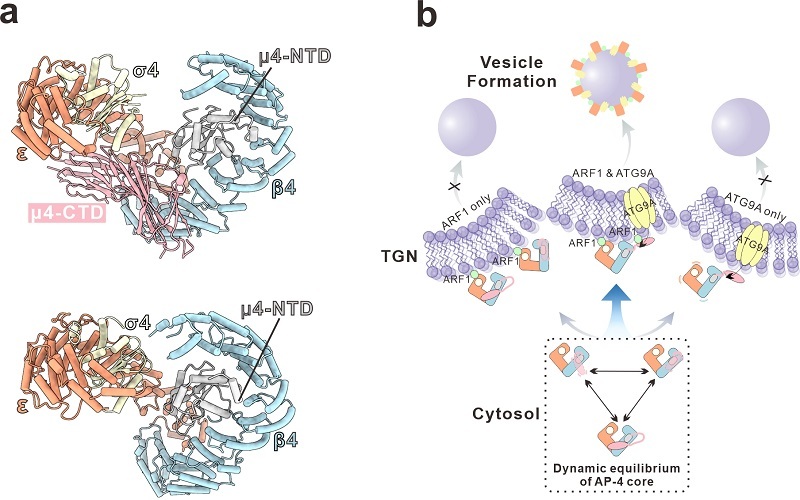
Cryo-EM structure of the AP-4 core complex and mechanistic model of its membrane recruitment (Image by FENG Wei’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202601/t20260123_1146486.shtml
- https://www.nature.com/articles/s41467-026-68679-8
AP-4の動的構造とARF1との関連性の構造的基礎 Structural basis for the dynamic conformations of AP-4 and its association with ARF1
Yanghui Wang,Wei Li,Yunlong Qiu,Si Wu,Liu Hong,Yan Zhao & Wei Feng
Nature Communications Published:21 January 2026
DOI:https://doi.org/10.1038/s41467-026-68679-8
We are providing an unedited version of this manuscript to give early access to its findings. Before final publication, the manuscript will undergo further editing. Please note there may be errors present which affect the content, and all legal disclaimers apply.
Abstract
Among the distinct adaptor protein (AP) complexes, AP-4 primarily functions as a non-clathrin-coated vesicle machinery essential for intracellular membrane trafficking. ARF1 is a master regulator of AP-4 membrane recruitment, but the underlying mechanism remains elusive. Here, we present the cryo-EM structures of soluble AP-4 and the AP-4/ARF1 complex. Unexpectedly, AP-4 adopts a dynamic equilibrium between closed and open conformations, caused by loose contacts between its medium subunit and central core. ARF1 binding induces only subtle changes in AP-4, which retains its conformational equilibrium. Mutations at the AP-4/ARF1 interface disrupt complex formation and impair ARF1-dependent membrane recruitment. Efficient membrane recruitment of AP-4 likely requires the synergistic engagement of ARF1 and cargoes. Disrupting the conformational flexibility of AP-4 interferes with this synergistic effect and compromises AP-4-mediated membrane trafficking. Our findings may redefine AP-4 as a conformationally dynamic complex modulated by cooperative interactions, providing insights into neurodevelopmental disorders associated with AP-4 dysfunction.