キネシン1というモータータンパク質の停止スイッチを発見 Scientists identify a stop switch for a motor protein called kinesin 1
2022-01-20 バッファロー大学(UB)

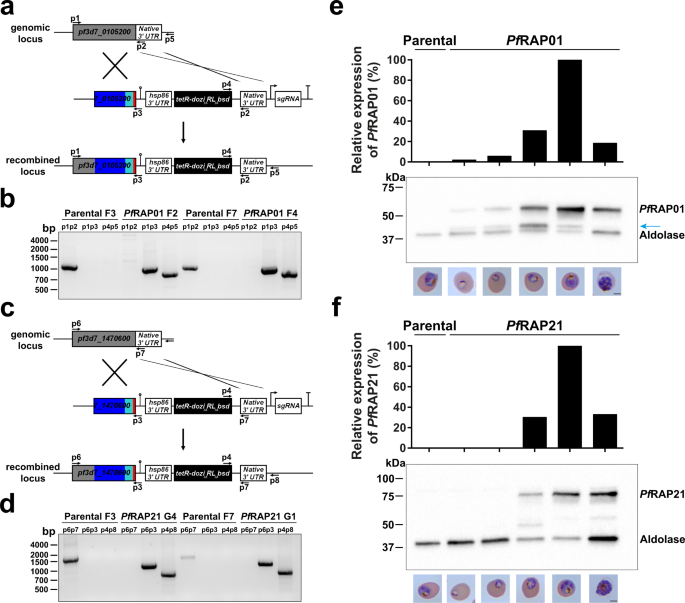
ある研究では、GSK3βと呼ばれる酵素がキネシン1と呼ばれるモータータンパク質の停止スイッチとしてどのように機能するかを説明しています。左のパネルの暗い線は、正常な動きのキネシン1モータータンパク質の軌跡を示しています。右のパネルの黒い点は、運動が停止しているキネシン1モータータンパク質の軌跡を示しています。Credit: Rupkatha Banerjee, adapted from a figure published in Development in a Dec. 23, 2021 article by Banerjee et al.
<関連情報>
- https://www.buffalo.edu/news/releases/2022/01/018.html
- https://journals.biologists.com/dev/article-abstract/148/24/dev199866/273844/A-stop-or-go-switch-glycogen-synthase-kinase-3?redirectedFrom=fulltext
キネシン1モータードメインのGlycogen synthase kinase 3βによる314番目のリン酸化は、微小管から切り離されずに運動を停止させる。 A stop or go switch: glycogen synthase kinase 3β phosphorylation of the kinesin 1 motor domain at Ser314 halts motility without detaching from microtubules
Rupkatha Banerjee, Piyali Chakraborty, Michael C. Yu, Shermali Gunawardena
Development (2021) 148 (24): dev199866. https://doi.org/10.1242/dev.199866
ABSTRACT
It is more than 25 years since the discovery that kinesin 1 is phosphorylated by several protein kinases. However, fundamental questions still remain as to how specific protein kinase(s) contribute to particular motor functions under physiological conditions. Because, within an whole organism, kinase cascades display considerable crosstalk and play multiple roles in cell homeostasis, deciphering which kinase(s) is/are involved in a particular process has been challenging. Previously, we found that GSK3β plays a role in motor function. Here, we report that a particular site on kinesin 1 motor domain (KHC), S314, is phosphorylated by GSK3β in vivo. The GSK3β-phosphomimetic-KHCS314D stalled kinesin 1 motility without dissociating from microtubules, indicating that constitutive GSK3β phosphorylation of the motor domain acts as a STOP. In contrast, uncoordinated mitochondrial motility was observed in CRISPR/Cas9-GSK3β non-phosphorylatable-KHCS314A Drosophila larval axons, owing to decreased kinesin 1 attachment to microtubules and/or membranes, and reduced ATPase activity. Together, we propose that GSK3β phosphorylation fine-tunes kinesin 1 movement in vivo via differential phosphorylation, unraveling the complex in vivo regulatory mechanisms that exist during axonal motility of cargos attached to multiple kinesin 1 and dynein motors.