2022-04-12 ペンシルベニア州立大学(PennState)
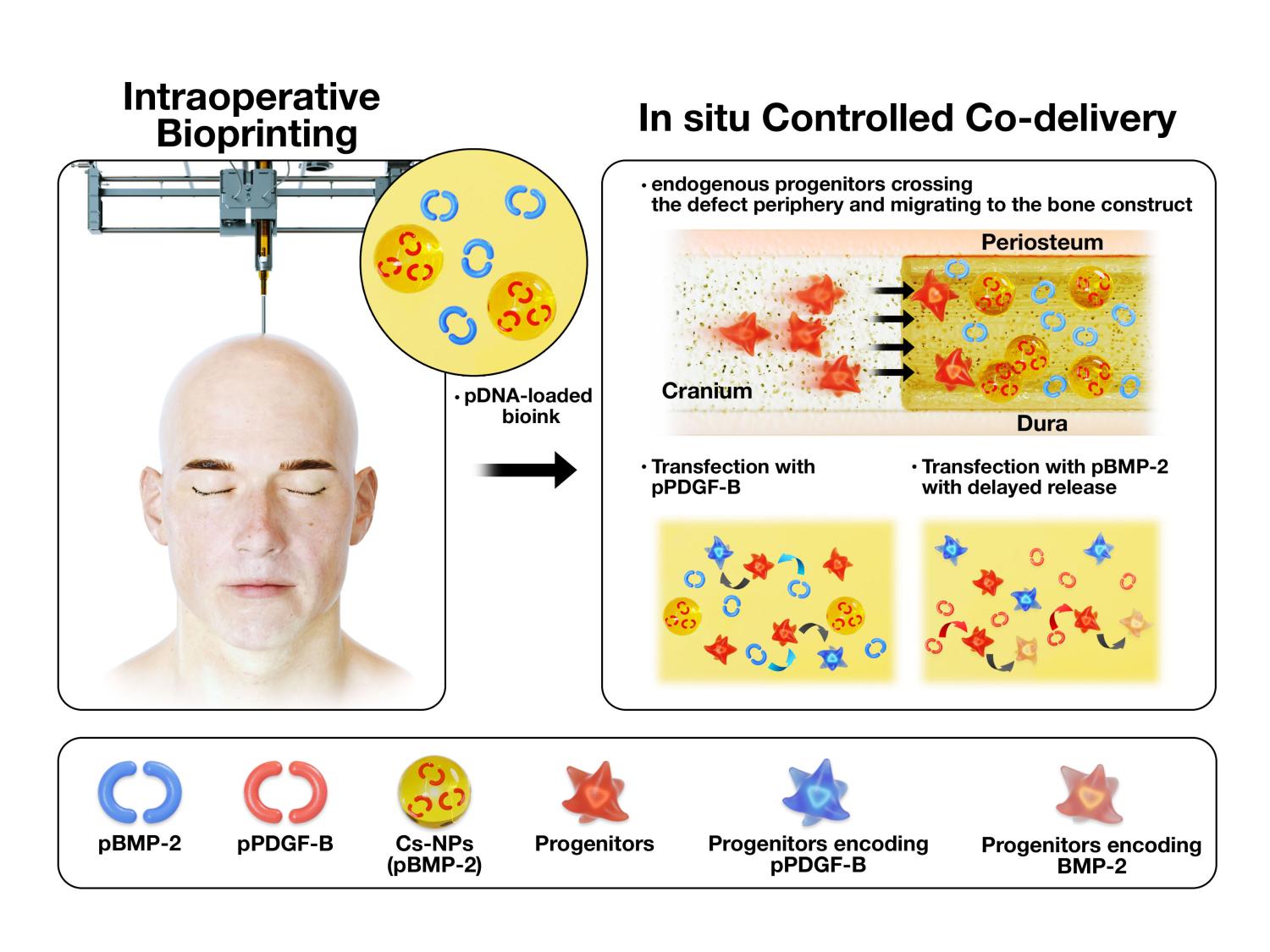 頭蓋骨欠損の修復のための制御された遺伝子共投与プラットフォームとして使用される骨構築物の手術中のバイオプリンティング。Credit: Dong Heo, Kyung Hee University; Ozbolat Lab, Penn
頭蓋骨欠損の修復のための制御された遺伝子共投与プラットフォームとして使用される骨構築物の手術中のバイオプリンティング。Credit: Dong Heo, Kyung Hee University; Ozbolat Lab, Penn
「成長因子は、細胞の増殖に不可欠なものです。私たちは、2つの異なる成長因子をコード化した2つの異なる遺伝子を使用しています。これらの成長因子は、幹細胞が欠損部分に移動するのを助け、その後、前駆細胞が骨に変換するのを助けてくれます。」と研究者は言う。
研究者達は、PDGF-B、血小板由来成長因子をコードする遺伝子を使い、これは、細胞の増殖と移動を促し、BMP-2、骨形成タンパク質をコードする遺伝子は、骨再生を向上させるものです。この2つの遺伝子は、バイオプリンティングという手法で埋め込まれた。
<関連情報>
- https://www.psu.edu/news/research/story/bioprinting-bone-repair-improved-genes/
- https://www.sciencedirect.com/science/article/abs/pii/S014296122100689X?via%3Dihub
術中に作製した骨コンストラクトからpPDGF-BとpBMP-2を制御して共送することにより、ラットの頭蓋窩欠損の修復が改善されること Controlled Co-delivery of pPDGF-B and pBMP-2 from intraoperatively bioprinted bone constructs improves the repair of calvarial defects in rats
Kazim K.Moncal,R. SedaTigli Aydın,Kevin P.Godzik,Timothy M.Acri,Dong N.Heo,EliasRizk,HwabokWee,Gregory S.Lewis,Aliasger K.Salem,Ibrahim T.Ozbolat
Science Direct
Published:28 December 2021
https://doi.org/10.1016/j.biomaterials.2021.121333
Abstract
Intraoperative bioprinting (IOB), which refers to the bioprinting process performed on a live subject in a surgical setting, has made it feasible to directly deliver gene-activated matrices into craniomaxillofacial (CMF) defect sites. In this study, we demonstrated a novel approach to overcome the current limitations of traditionally fabricated non-viral gene delivery systems through direct IOB of bone constructs into defect sites. We used a controlled co-delivery release of growth factors from a gene-activated matrix (an osteogenic bioink loaded with plasmid-DNAs (pDNA)) to promote bone repair. The controlled co-delivery approach was achieved from the combination of platelet-derived growth factor-B encoded plasmid-DNA (pPDGF-B) and chitosan-nanoparticle encapsulating pDNA encoded with bone morphogenetic protein-2 (CS-NPs(pBMP2)), which facilitated a burst release of pPDGF-B in 10 days, and a sustained release of pBMP-2 for 5 weeks in vitro. The controlled co-delivery approach was tested for its potential to repair critical-sized rat calvarial defects. The controlled-released pDNAs from the intraoperatively bioprinted bone constructs resulted in ∼40% bone tissue formation and ∼90% bone coverage area at 6 weeks compared to ∼10% new bone tissue and ∼25% total bone coverage area in empty defects. The delivery of growth factors incorporated within the intraoperatively bioprinted constructs could pose as an effective way to enhance bone regeneration in patients with cranial injuries in the future.