2023-09-28 アイルランド・リムリック大学(UL)
◆精密医療は、個々の腫瘍の特性をターゲットにした治療を提供し、腫瘍の縮小率や無増悪生存期を改善する可能性が高いとされています。この研究は、精密医療アプローチを採用することで、治療の成功率が向上し、同時にコストが削減されることを示唆しており、がん患者に手頃な医療ケアを提供する新たな道を開いています。
<関連情報>
- https://www.ul.ie/news/landmark-study-involving-ul-research-demonstrates-economic-benefit-of-precision-medicine-in
- https://joppp.biomedcentral.com/articles/10.1186/s40545-023-00590-9
プレシジョン・オンコロジー・パラダイムの実現:コンパニオン診断薬を活用したプレシジョン・オンコロジー医薬品アプローチによる研究開発コストの削減と投資収益率の向上 Delivering the precision oncology paradigm: reduced R&D costs and greater return on investment through a companion diagnostic informed precision oncology medicines approach
Raymond H. Henderson,Declan French,Elaine Stewart,Dave Smart,Adam Idica,Sandra Redmond,Markus Eckstein,Jordan Clark,Richard Sullivan,Peter Keeling & Mark Lawler
Journal of Pharmaceutical Policy and Practice Published:05 July 2023
DOI:https://doi.org/10.1186/s40545-023-00590-9
Abstract
Background
Precision oncology medicines represent a paradigm shift compared to non-precision oncology medicines in cancer therapy, in some situations delivering more clinical benefit, and potentially lowering healthcare costs. We determined whether employing a companion diagnostic (CDx) approach during oncology medicines development delivers effective therapies that are within the cost constraints of current health systems. R&D costs of developing a medicine are subject to debate, with average estimates ranging from $765 million (m) to $4.6 billion (b). Our aim was to determine whether precision oncology medicines are cheaper to bring from R&D to market; a secondary goal was to determine whether precision oncology medicines have a greater return on investment (ROI).
Method
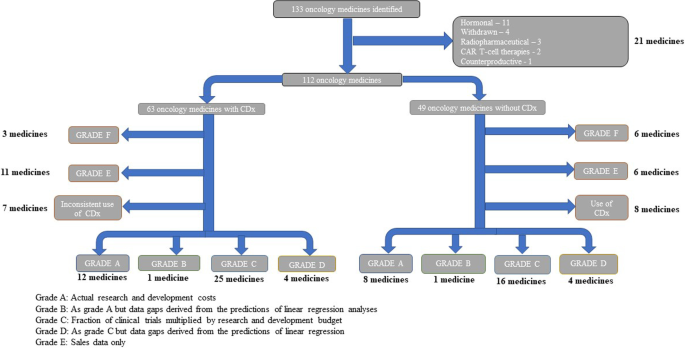
Data on oncology medicines approved between 1997 and 2020 by the US Food and Drug Administration (FDA) were analysed from the Securities and Exchange Commission (SEC) filings. Data were compiled from 10-K, 10-Q, and 20-F financial performance filings on medicines’ development costs through their R&D lifetime. Clinical trial data were split into clinical trial phases 1–3 and probability of success (POS) of trials was calculated, along with preclinical costs. Cost-of-capital (CoC) approach was applied and, if appropriate, a tax rebate was subtracted from the total.
Results
Data on 42 precision and 29 non-precision oncology medicines from 56 companies listed by the National Cancer Institute which had complete data available were analysed. Estimated mean cost to deliver a new oncology medicine was $4.4b (95% CI, $3.6–5.2b). Costs to bring a precision oncology medicine to market were $1.1b less ($3.5b; 95% CI, $2.7–4.5b) compared to non-precision oncology medicines ($4.6b; 95% CI, $3.5–6.1b). The key driver of costs was POS of clinical trials, accounting for a difference of $591.3 m. Additional data analysis illustrated that there was a 27% increase in return on investment (ROI) of precision oncology medicines over non-precision oncology medicines.
Conclusion
Our results provide an accurate estimate of the R&D spend required to bring an oncology medicine to market. Deployment of a CDx at the earliest stage substantially lowers the cost associated with oncology medicines development, potentially making them available to more patients, while staying within the cost constraints of cancer health systems.