2023-10-10 エディンバラ大学
◆この研究は、鳥のポピュレーションを保護し、人間と野生の鳥へのリスクを減少させる可能性があります。遺伝子編集は鳥インフルエンザに対する希望に満ちたアプローチですが、さらなる研究が必要です。
<関連情報>
- https://www.ed.ac.uk/news/2023/gene-edited-chickens-in-fight-against-bird-flu
- https://www.nature.com/articles/s41467-023-41476-3
ANP32遺伝子ファミリーのゲノム編集により鳥インフルエンザ感染に対する抵抗性を創出 Creating resistance to avian influenza infection through genome editing of the ANP32 gene family
Alewo Idoko-Akoh,Daniel H. Goldhill,Carol M. Sheppard,Dagmara Bialy,Jessica L. Quantrill,Ksenia Sukhova,Jonathan C. Brown,Samuel Richardson,Ciara Campbell,Lorna Taylor,Adrian Sherman,Salik Nazki,Jason S. Long,Michael A. Skinner,Holly Shelton,Helen M. Sang,Wendy S. Barclay & Mike J. McGrew
Nature Communications Published:10 October 2023
DOI:https://doi.org/10.1038/s41467-023-41476-3
Abstract
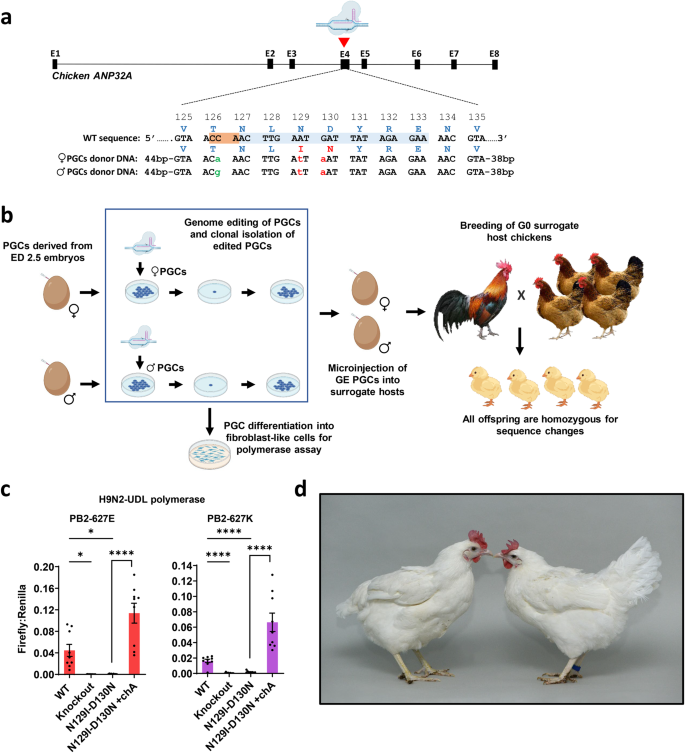
Chickens genetically resistant to avian influenza could prevent future outbreaks. In chickens, influenza A virus (IAV) relies on host protein ANP32A. Here we use CRISPR/Cas9 to generate homozygous gene edited (GE) chickens containing two ANP32A amino acid substitutions that prevent viral polymerase interaction. After IAV challenge, 9/10 edited chickens remain uninfected. Challenge with a higher dose, however, led to breakthrough infections. Breakthrough IAV virus contained IAV polymerase gene mutations that conferred adaptation to the edited chicken ANP32A. Unexpectedly, this virus also replicated in chicken embryos edited to remove the entire ANP32A gene and instead co-opted alternative ANP32 protein family members, chicken ANP32B and ANP32E. Additional genome editing for removal of ANP32B and ANP32E eliminated all viral growth in chicken cells. Our data illustrate a first proof of concept step to generate IAV-resistant chickens and show that multiple genetic modifications will be required to curtail viral escape.