0023-11-08 カリフォルニア大学リバーサイド校(UCR)
◆Nature誌に掲載された新しい論文では、CAGリピートのRNAメチル化が、ハンチントン病の根底にある複雑なメカニズムにどのように関与しているかが詳述されています。また、研究者らがミミズやショウジョウバエの病気の進行を大幅に抑制し、メチル化を除去するタンパク質を細胞に導入することでハエの寿命を延ばした方法についても説明しています。
◆現在のところ、ハンチントン病を治す方法や進行を遅らせる方法はありません。医療提供者は通常、いくつかの症状を助けるために薬を提供します。このブレークスルーは治療法ではありませんが、現在存在しない効果的な治療法の可能性を示しています。
<関連情報>
- https://news.ucr.edu/articles/2023/11/08/scientists-tame-biological-trigger-deadly-huntingtons-disease
- https://www.nature.com/articles/s41586-023-06701-5
CAGリピートRNAのm1AはTDP-43に結合し、神経変性を誘導する m1A in CAG repeat RNA binds to TDP-43 and induces neurodegeneration
Yuxiang Sun,Hui Dai,Xiaoxia Dai,Jiekai Yin,Yuxiang Cui,Xiaochuan Liu,Gwendolyn Gonzalez,Jun Yuan,Feng Tang,Nan Wang,Alexandra E. Perlegos,Nancy M. Bonini,X. William Yang,Weifeng Gu & Yinsheng Wang
Nature Published:08 November 2023
DOI:https://doi.org/10.1038/s41586-023-06701-5
Abstract
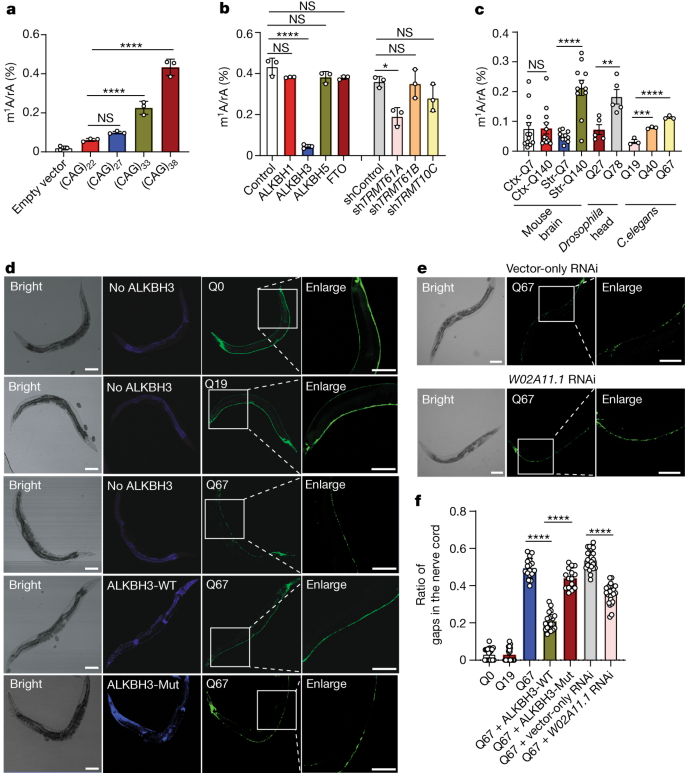
Microsatellite repeat expansions within genes contribute to a number of neurological diseases1,2. The accumulation of toxic proteins and RNA molecules with repetitive sequences, and/or sequestration of RNA-binding proteins by RNA molecules containing expanded repeats are thought to be important contributors to disease aetiology3,4,5,6,7,8,9. Here we reveal that the adenosine in CAG repeat RNA can be methylated to N1-methyladenosine (m1A) by TRMT61A, and that m1A can be demethylated by ALKBH3. We also observed that the m1A/adenosine ratio in CAG repeat RNA increases with repeat length, which is attributed to diminished expression of ALKBH3 elicited by the repeat RNA. Additionally, TDP-43 binds directly and strongly with m1A in RNA, which stimulates the cytoplasmic mis-localization and formation of gel-like aggregates of TDP-43, resembling the observations made for the protein in neurological diseases. Moreover, m1A in CAG repeat RNA contributes to CAG repeat expansion-induced neurodegeneration in Caenorhabditis elegans and Drosophila. In sum, our study offers a new paradigm of the mechanism through which nucleotide repeat expansion contributes to neurological diseases and reveals a novel pathological function of m1A in RNA. These findings may provide an important mechanistic basis for therapeutic intervention in neurodegenerative diseases emanating from CAG repeat expansion.