栄養失調の子どもたちの治療に有益なバクテリアの存在を示すパイロット試験結果 Pilot study provides more evidence beneficial bacteria could help treat malnourished children
2022-04-13 ワシントン大学セントルイス
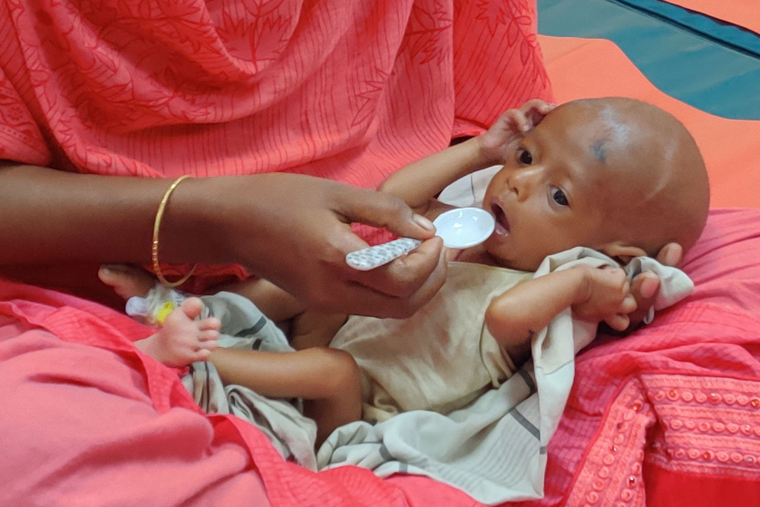
A new study led by Washington University School of Medicine in St. Louis and the International Centre for Diarrhoeal Disease Research in Dhaka, Bangladesh, shows that a standard milk-based therapy plus treatment with a specific strain of gut bacteria known as Bifidobacterium infantis (B. infantis) reduces gut inflammation and promotes weight gain in infants with severe acute malnutrition. Pictured is a mother feeding her infant, who has severe acute malnutrition. (Photo: Ridwan Islam, International Centre for Diarrhoeal Disease Research, Bangladesh)
<関連情報>
- https://source.wustl.edu/2022/04/gut-bacterium-supports-growth-in-infants-with-severe-acute-malnutrition/
- https://www.science.org/doi/10.1126/scitranslmed.abk1107
バングラデシュの重症急性栄養失調児におけるビフィズス菌治療による体重増加の促進について Bifidobacterium infantis treatment promotes weight gain in Bangladeshi infants with severe acute malnutrition
MICHAEL J. BARRATT,SHARIKA NUZHAT,KAZI AHSAN,STEVEN A. FRESE,ALEKSANDR A. ARZAMASOV ,SHAFIQUL ALAM SARKER,M. MUNIRUL ISLAM,PARAG PALIT,MD RIDWAN ISLAM ,MATTHEW C. HIBBERD,SWETHA NAKSHATRI,CARRIE A. COWARDIN,JANAKI L. GURUGE,ALEXANDRA E. BYRNE,SIDDARTH VENKATESH,VINAIK SUNDARESAN,BETHANY HENRICK ,REBBECA M. DUAR,RYAN D. MITCHELL,GIORGIO CASABURI,JOHANN PRAMBS ,ROBIN FLANNERY,MUSTAFA MAHFUZ,DMITRY A. RODIONOV,ANDREI L. OSTERMAN ,DAVID KYLE ,TAHMEED AHMED AND JEFFREY I. GORDON
Science Translational Medicine Published:13 Apr 2022
DOI: 10.1126/scitranslmed.abk1107
Abstract
Disrupted development of the gut microbiota is a contributing cause of childhood malnutrition. Bifidobacterium longum subspecies infantis is a prominent early colonizer of the infant gut that consumes human milk oligosaccharides (HMOs). We found that the absolute abundance of Bifidobacterium infantis is lower in 3- to 24-month-old Bangladeshi infants with severe acute malnutrition (SAM) compared to their healthy age-matched counterparts. A single-blind, placebo-controlled trial (SYNERGIE) was conducted in 2- to 6-month-old Bangladeshi infants with SAM. A commercial U.S. donor–derived B. infantis strain (EVC001) was administered daily with or without the HMO lacto-N-neotetraose for 28 days. This intervention increased fecal B. infantis abundance in infants with SAM, although to levels still 10- to 100-fold lower than in untreated healthy controls. EVC001 treatment promoted weight gain that was associated with reduced intestinal inflammation markers in infants with SAM. We cultured fecal B. infantis strains from Bangladeshi infants and colonized gnotobiotic mice with these cultured strains. The gnotobiotic mice were fed a diet representative of that consumed by 6-month-old Bangladeshi infants, with or without HMO supplementation. One B. infantis strain, Bg_2D9, expressing two gene clusters involved in uptake and utilization of N-glycans and plant-derived polysaccharides, exhibited superior fitness over EVC001. The fitness advantage of Bg_2D9 was confirmed in a gnotobiotic mouse model of mother-to-infant gut microbiota transmission where dams received a pretreatment fecal community from a SAM infant in the SYNERGIE trial. Whether Bg_2D9 is superior to EVC001 for treating malnourished infants who consume a diet with limited breastmilk requires further clinical testing.