2024-03-08 カロリンスカ研究所(KI)
<関連情報>
- https://news.ki.se/study-shows-how-oestrogen-protects-against-fatty-liver
- https://www.embopress.org/doi/full/10.1038/s44320-024-00024-x
エストロゲン受容体活性化がTEAD1遺伝子発現をリモデリングし肝脂肪症を緩和する Estrogen receptor activation remodels TEAD1 gene expression to alleviate hepatic steatosis
Christian Sommerauer , Carlos J Gallardo-Dodd, Christina Savva, Linnea Hases, Madeleine Birgersson, Rajitha Indukuri, Joanne X Shen , Pablo Carravilla, Keyi Geng, Jonas Nørskov Søndergaard , Clàudia Ferrer-Aumatell, Grégoire Mercier, Erdinc Sezgin , Marion Korach-André, Carl Petersson, Hannes Hagström, Volker M Lauschke , Amena Archer , Cecilia Williams, and Claudia Kutter
Molecular Systems Biology published:8 March 2024
DOI:https://doi.org/10.1038/s44320-024-00024-x
Abstract
Sex-based differences in obesity-related hepatic malignancies suggest the protective roles of estrogen. Using a preclinical model, we dissected estrogen receptor (ER) isoform-driven molecular responses in high-fat diet (HFD)-induced liver diseases of male and female mice treated with or without an estrogen agonist by integrating liver multi-omics data. We found that selective ER activation recovers HFD-induced molecular and physiological liver phenotypes. HFD and systemic ER activation altered core liver pathways, beyond lipid metabolism, that are consistent between mice and primates. By including patient cohort data, we uncovered that ER-regulated enhancers govern central regulatory and metabolic genes with clinical significance in metabolic dysfunction-associated steatotic liver disease (MASLD) patients, including the transcription factor TEAD1. TEAD1 expression increased in MASLD patients, and its downregulation by short interfering RNA reduced intracellular lipid content. Subsequent TEAD small molecule inhibition improved steatosis in primary human hepatocyte spheroids by suppressing lipogenic pathways. Thus, TEAD1 emerged as a new therapeutic candidate whose inhibition ameliorates hepatic steatosis.
Synopsis
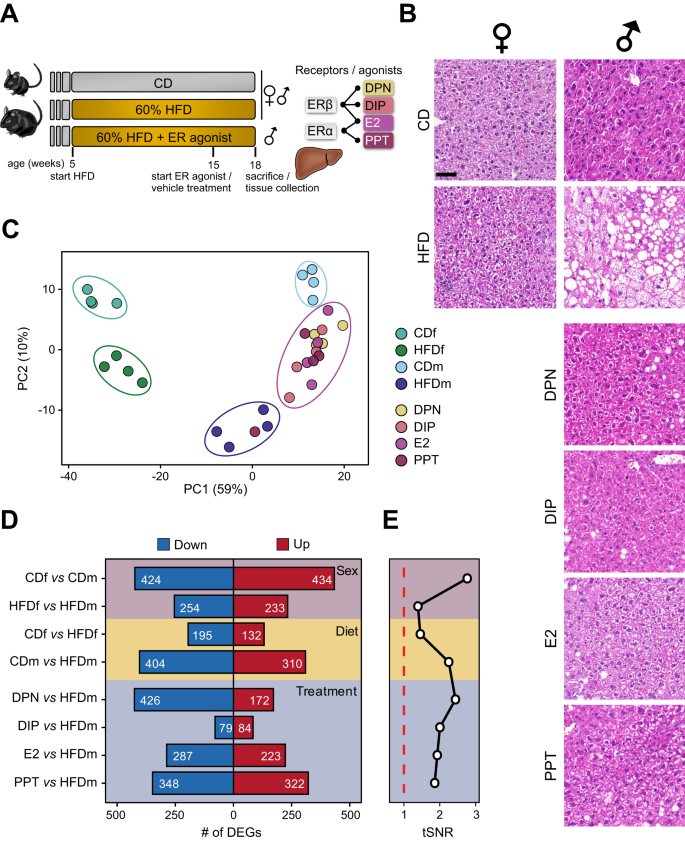
Integration of genomics datasets and patient data reveals estrogen receptor agonist-mediated restoration of molecular and physiological changes and identifies a TEAD autopalmitoylation inhibitor as a potential therapeutic for metabolic dysfunction-associated steatotic liver disease (MASLD).
•Selective activation of nuclear ERs recovers high fat diet (HFD)-induced molecular and physiological liver phenotypes, whereby ERβ represses fibrogenic factors.
•HFD and systemic ER activation alter core liver pathways, beyond lipid metabolism, that are maintained between mice and primates.
•ER-sensitive enhancers govern central regulatory and metabolic genes with significance in MASLD patients, including TEAD1.
•TEAD inhibition improves steatosis in primary human hepatocyte spheroids by suppressing lipogenic pathways.


