2024-03-25 バース大学
<関連情報>
- https://www.bath.ac.uk/announcements/cbd-products-dont-ease-pain-and-are-potentially-harmful-new-study-finds/
- https://www.jpain.org/article/S1526-5900(23)00582-5/fulltext
痛みのためのカンナビジオール(CBD)製品:効果がなく、高価で、潜在的な害がある Cannabidiol (CBD) Products for Pain: Ineffective, Expensive, and With Potential Harms
Andrew Moore,Sebastian Straube,Emma Fisher,Christopher Eccleston
The Journal of Pain Published:October 18, 2023
DOI:https://doi.org/10.1016/j.jpain.2023.10.009
Highlights
•Cannabidiol (CBD) products have varying amounts of CBD, from none to much more than advertised.
•CBD products may contain other chemicals than CBD, some of which may be harmful.
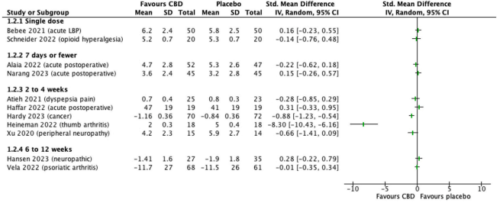
•Sixteen RCTs for pain used pharmaceutical CBD in oral, buccal/sublingual, and topical forms.
•Fifteen of the 16 RCTs were negative: no greater pain-relieving effect for CBD than for placebo.
•Meta-analyses link CBD to increased rates of serious adverse events and hepatotoxicity.
Abstract
Cannabidiol (CBD) attracts considerable attention for promoting good health and treating various conditions, predominantly pain, often in breach of advertising rules. Examination of available CBD products in North America and Europe demonstrates that CBD content can vary from none to much more than advertised and that potentially harmful other chemicals are often included. Serious harm is associated with chemicals found in CBD products and reported in children, adults, and the elderly. A 2021 International Association for the Study of Pain task force examined the evidence for cannabinoids and pain but found no trials of CBD. Sixteen CBD randomized trials using pharmaceutical-supplied CBD or making preparations from such a source and with pain as an outcome have been published subsequently. The trials were conducted in 12 different pain states, using 3 oral, topical, and buccal/sublingual administration, with CBD doses between 6 and 1,600 mg, and durations of treatment between a single dose and 12 weeks. Fifteen of the 16 showed no benefit of CBD over placebo. Small clinical trials using verified CBD suggest the drug to be largely benign; while large-scale evidence of safety is lacking, there is growing evidence linking CBD to increased rates of serious adverse events and hepatotoxicity. In January 2023, the Food and Drug Administration (FDA) announced that a new regulatory pathway for CBD was needed. Consumers and health care providers should rely on evidence-based sources of information on CBD, not just advertisements. Current evidence is that CBD for pain is expensive, ineffective, and possibly harmful.
Perspective
There is no good reason for thinking that CBD relieves pain, but there are good reasons for doubting the contents of CBD products in terms of CBD content and purity.