2024-04-15 ワシントン大学セントルイス校
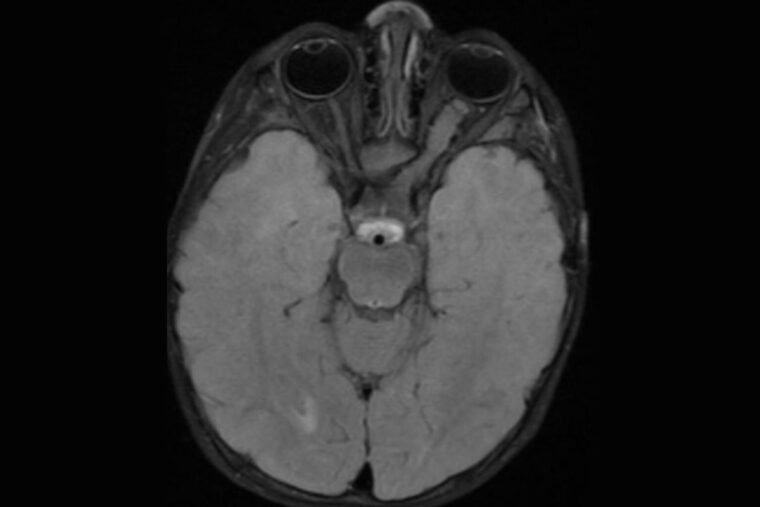
A brain scan of a neurofibromatosis type 1 (NF1) patient reveals a tumor on the optic nerve connecting the left eye to the brain (right side of the image). Researchers at Washington University School of Medicine in St. Louis have discovered that an FDA-approved epilepsy drug can prevent or slow the growth of NF1-linked optic gliomas in mice, laying the groundwork for a clinical trial. (Image: Robert McKinstry/School of Medicine)
<関連情報>
- https://source.wustl.edu/2024/04/epilepsy-drug-prevents-brain-tumors-in-mice-with-nf1/
- https://academic.oup.com/neuro-oncology/advance-article-abstract/doi/10.1093/neuonc/noae054/7637150?redirectedFrom=fulltext
NF1変異による神経細胞の興奮亢進が、マウス視神経膠腫の腫瘍形成と治療標的の閾値を設定する NF1 mutation-driven neuronal hyperexcitability sets a threshold for tumorigenesis and therapeutic targeting of murine optic glioma
Corina Anastasaki, Jit Chatterjee, Joshua P Koleske, Yunqing Gao, Stephanie L Bozeman, Chloe M Kernan, Lara I Marco Y Marquez, Ji-Kang Chen, Caitlin E Kelly, Connor J Blair …
Neuro-Oncology Published:12 April 2024
DOI:https://doi.org/10.1093/neuonc/noae054
Abstract
Background
With the recognition that noncancerous cells function as critical regulators of brain tumor growth, we recently demonstrated that neurons drive low-grade glioma initiation and progression. Using mouse models of neurofibromatosis type 1 (NF1)-associated optic pathway glioma (OPG), we showed that Nf1 mutation induces neuronal hyperexcitability and midkine expression, which activates an immune axis to support tumor growth, such that high-dose lamotrigine treatment reduces Nf1-OPG proliferation. Herein, we execute a series of complementary experiments to address several key knowledge gaps relevant to future clinical translation.
Methods
We leverage a collection of Nf1-mutant mice that spontaneously develop OPGs to alter both germline and retinal neuron-specific midkine expression. Nf1-mutant mice harboring several different NF1 patient-derived germline mutations were employed to evaluate neuronal excitability and midkine expression. Two distinct Nf1-OPG preclinical mouse models were used to assess lamotrigine effects on tumor progression and growth in vivo.
Results
We establish that neuronal midkine is both necessary and sufficient for Nf1-OPG growth, demonstrating an obligate relationship between germline Nf1 mutation, neuronal excitability, midkine production, and Nf1-OPG proliferation. We show anti-epileptic drug (lamotrigine) specificity in suppressing neuronal midkine production. Relevant to clinical translation, lamotrigine prevents Nf1-OPG progression and suppresses the growth of existing tumors for months following drug cessation. Importantly, lamotrigine abrogates tumor growth in two Nf1-OPG strains using pediatric epilepsy clinical dosing.
Conclusions
Together, these findings establish midkine and neuronal hyperexcitability as targetable drivers of Nf1-OPG growth and support the use of lamotrigine as a potential chemoprevention or chemotherapy agent for children with NF1-OPG.


