2024-06-17 デューク大学(Duke)
<関連情報>
- https://pratt.duke.edu/news/protein-based-homing-device-precisely-delivers-drugs-to-target-neurons/
- https://www.nature.com/articles/s41592-024-02292-9
DART.2:千倍の細胞特異性を持つ双方向シナプス薬理学 DART.2: bidirectional synaptic pharmacology with thousandfold cellular specificity
Brenda C. Shields,Haidun Yan,Shaun S. X. Lim,Sasha C. V. Burwell,Celine M. Cammarata,Elizabeth A. Fleming,S. Aryana Yousefzadeh,Victoria Z. Goldenshtein,Elizabeth W. Kahuno,Purav P. Vagadia,Marie H. Loughran,Lei Zhiquan,Mark E. McDonnell,Miranda L. Scalabrino,Mishek Thapa,Tammy M. Hawley,Greg D. Field,Court Hull,Gary E. Schiltz,Lindsey L. Glickfeld,Allen B. Reitz & Michael R. Tadross
Nature Methods Published:14 June 2024
DOI:https://doi.org/10.1038/s41592-024-02292-9
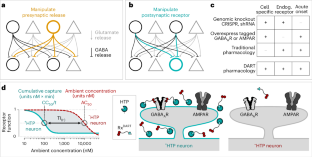
Abstract
Precision pharmacology aims to manipulate specific cellular interactions within complex tissues. In this pursuit, we introduce DART.2 (drug acutely restricted by tethering), a second-generation cell-specific pharmacology technology. The core advance is optimized cellular specificity—up to 3,000-fold in 15 min—enabling the targeted delivery of even epileptogenic drugs without off-target effects. Additionally, we introduce brain-wide dosing methods as an alternative to local cannulation and tracer reagents for brain-wide dose quantification. We describe four pharmaceuticals—two that antagonize excitatory and inhibitory postsynaptic receptors, and two that allosterically potentiate these receptors. Their versatility is showcased across multiple mouse-brain regions, including cerebellum, striatum, visual cortex and retina. Finally, in the ventral tegmental area, we find that blocking inhibitory inputs to dopamine neurons accelerates locomotion, contrasting with previous optogenetic and pharmacological findings. Beyond enabling the bidirectional perturbation of chemical synapses, these reagents offer intersectional precision—between genetically defined postsynaptic cells and neurotransmitter-defined presynaptic partners.