2024-12-03 インペリアル・カレッジ・ロンドン(ICL)
<関連情報>
- https://www.imperial.ac.uk/news/258953/chemical-metronome-helps-brain-keep-time/
- https://www.embopress.org/doi/full/10.1038/s44318-024-00324-w
- https://www.science.org/doi/10.1126/science.aat4104
視交叉上核におけるアストロサイトGABAのリズミカルな産生が神経細胞の概日リズムを同期させる Rhythmic astrocytic GABA production synchronizes neuronal circadian timekeeping in the suprachiasmatic nucleus
Natalie Ness, Sandra Díaz-Clavero, Marieke M B Hoekstra, and Marco Brancaccio
The EMBO Journal Published:2 December 2024
DOI:https://doi.org/10.1038/s44318-024-00324-w
Abstract
Astrocytes of the suprachiasmatic nucleus (SCN) can regulate sleep-wake cycles in mammals. However, the nature of the information provided by astrocytes to control circadian patterns of behavior is unclear. Neuronal circadian activity across the SCN is organized into spatiotemporal waves that govern seasonal adaptations and timely engagement of behavioral outputs. Here, we show that astrocytes across the mouse SCN exhibit instead a highly uniform, pulse-like nighttime activity. We find that rhythmic astrocytic GABA production via polyamine degradation provides an inhibitory nighttime tone required for SCN circuit synchrony, thereby acting as an internal astrocyte zeitgeber (or “astrozeit”). We further identify synaptic GABA and astrocytic GABA as two key players underpinning coherent spatiotemporal circadian patterns of SCN neuronal activity. In describing a new mechanism by which astrocytes contribute to circadian timekeeping, our work provides a general blueprint for understanding how astrocytes encode temporal information underlying complex behaviors in mammals.
Synopsis
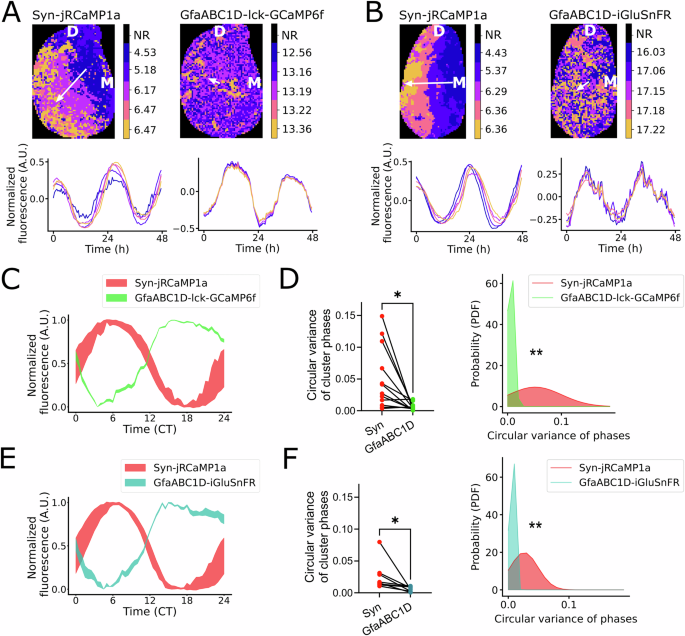
Astrocytes can drive circadian behavior in mammals, but the nature of the temporal information generated by astrocytes is largely unknown. This study identifies GABA produced by polyamine degradation in astrocytes as a critical signal that synchronizes neuronal activity in the suprachiasmatic nucleus (SCN), which orchestrates circadian rhythms in mammals.
•Circadian phases of astrocytic activity are uniform across the SCN, in contrast to the phase waves of neuronal activity.
•Extracellular GABA levels in the SCN peak during the nighttime, mirroring the spatiotemporal organization of astrocytic rhythms, as opposed to the daytime peak of neuronal activity.
•Inhibition of synaptic GABA release desynchronizes neuronal circadian rhythms in the SCN, but does not affect extracellular GABA rhythms.
•Inhibition of astrocytic GABA synthesis disrupts circadian rhythms of extracellular GABA and desynchronizes neuronal circadian activity, suggesting a role as an internal circadian synchronizer for the SCN circuit (“astrozeit”)
アストロサイトの細胞自律時計が哺乳類の概日行動を駆動する Cell-autonomous clock of astrocytes drives circadian behavior in mammals
Marco Brancaccio, Mathew D. Edwards, Andrew P. Patton, Nicola J. Smyllie, […], and Michael H. Hastings
Science Published:11 Jan 2019
DOI:https://doi.org/10.1126/science.aat4104
Astrocytes can drive the master clock in the brain
The neurons of the suprachiasmatic nucleus (SCN) of the hypothalamus function as a central circadian clock, coordinating mammalian physiology with the 24-hour light-dark cycle. Brancaccio et al. found that these neurons have help from neighboring astrocytes (see the Perspective by Green). In mice lacking the Cry gene, which encodes a critical clock component, restoration of Cry expression and molecular clock function in the astrocytes, but not the neighboring neurons, restored rhythmic transcriptional oscillations in the SCN and reestablished circadian behaviors in the mice.
Science, this issue p. 187; see also p. 124
Abstract
Circadian (~24-hour) rhythms depend on intracellular transcription-translation negative feedback loops (TTFLs). How these self-sustained cellular clocks achieve multicellular integration and thereby direct daily rhythms of behavior in animals is largely obscure. The suprachiasmatic nucleus (SCN) is the fulcrum of this pathway from gene to cell to circuit to behavior in mammals. We describe cell type–specific, functionally distinct TTFLs in neurons and astrocytes of the SCN and show that, in the absence of other cellular clocks, the cell-autonomous astrocytic TTFL alone can drive molecular oscillations in the SCN and circadian behavior in mice. Astrocytic clocks achieve this by reinstating clock gene expression and circadian function of SCN neurons via glutamatergic signals. Our results demonstrate that astrocytes can autonomously initiate and sustain complex mammalian behavior.