2025-02-21 シンガポール国立大学 (NUS)
<関連情報>
- https://news.nus.edu.sg/novel-method-synthesise-fluorinated-drug-compounds/
- https://www.nature.com/articles/s41557-024-01730-7
触媒的ジフルオロカルベン挿入によりフッ素化オキセタンイソステアへのアクセスが可能に Catalytic difluorocarbene insertion enables access to fluorinated oxetane isosteres
Tong-De Tan,Fang Zhou,Kevin P. Quirion,Yu-Qi Wang,Daniel Zhi Wei Ng,Xiaohua Luo,Eric Chun Yong Chan,Peng Liu & Ming Joo Ko
Nature Chemistry Published:20 February 2025
DOI:https://doi.org/10.1038/s41557-024-01730-7
Abstract
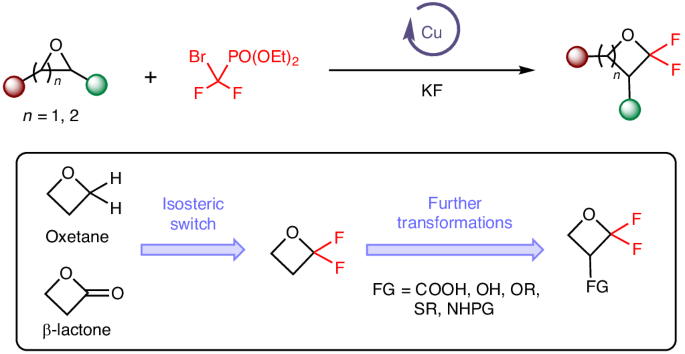
Skeletal editing of heterocyclic building blocks offers an appealing way to expand the accessible chemical space by diversifying molecular scaffolds for drug discovery. Despite the recent boom in this area, catalytic strategies that directly introduce fluorine into the backbone of small-ring heterocycles remain rare owing to the challenges of strain-induced ring cleavage and defluorination. Here we describe a copper-catalysed approach for skeletal expansion of oxygen heterocycles by reaction with a difluorocarbene species generated in situ to induce carbon atom insertion. The α,α-difluoro-oxetane products are potential surrogates of oxetane, β-lactone and carbonyl pharmacophores on the basis of their computed molecular properties and electrostatic potential maps. The utility of this approach is highlighted by synthesis of various drug-like molecules and fluorinated isosteres of biologically active compounds. Experimental and computational investigations provide insight into the mechanism and the unique role of the copper catalyst in promoting both ring-opening and cyclization steps of the reaction.