2025-08-21 マウントサイナイ医療システム(MSHS)
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/combination-therapy-improves-outcomes-for-advanced-triple-negative-breast-cancer
- https://link.springer.com/article/10.1007/s10549-025-07802-7
進行性トリプルネガティブ乳がんに対する単剤カルボプラチンとカルボプラチンとエベロリムスの組み合わせのランダム化第 II 相比較試験 Randomized phase II comparison of single-agent carboplatin versus combination of carboplatin and everolimus for advanced triple negative breast cancer
Rima Patel,Jami Fukui,Paula Klein,Erin Moshier,Hulya Kocyigit,Laura Fiedler,Weronika Bucwinska,Xiao Y. Xing,Charles Shapiro,Anupama Goel,Julie Fasano,Theresa Shao,Aarti Bhardwaj,Esther Kim,Rita Vaccaro,Karen Lee,Eric Wilck & Amy Tiersten
Breast Cancer Research and Treatment Published:16 August 2025
DOI:https://doi.org/10.1007/s10549-025-07802-7
Abstract
Purpose
Triple-negative breast cancers (TNBCs) are associated with a high frequency of PTEN loss, which can lead to activation of the mTOR pathway and tumor proliferation but may be reversible with the mTOR inhibitor everolimus. A prior phase II single-arm trial of carboplatin and everolimus in patients with advanced TNBC demonstrated good tolerability and preliminary efficacy.
Patients and methods
A phase II randomized trial in patients with advanced TNBC, with 0–3 prior lines of therapy, was conducted. Patients were randomized 2:1 to receive carboplatin and everolimus or carboplatin alone. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), overall response rate (ORR), clinical benefit rate (CBR), and safety.
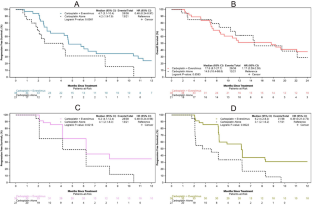
Results
Between 2015 and 2022, 59 patients were randomized to carboplatin/everolimus (n = 38) or carboplatin alone (n = 21). The median age of the population was 62 years and 68% had received at least one prior line of therapy. Median PFS was significantly improved in patients who received carboplatin/everolimus (4.7 months) versus carboplatin alone (4.2 months; HR:0.49; 95% CI: 0.25–0.98; p = 0.0390). OS was 17.6 months with the combination and 14.6 months with carboplatin alone (HR:1.17; 95% CI: 0.59–2.30; p = 0.6593). The most common adverse events (AEs) on the combination included thrombocytopenia, anemia, leukopenia, and neutropenia.
Conclusion
The combination of carboplatin and everolimus in this study reduced the risk of progression or death by 52%. The regimen was well tolerated and provides a promising treatment option for patients with advanced TNBC.