2025-09-03 ワシントン大学セントルイス校
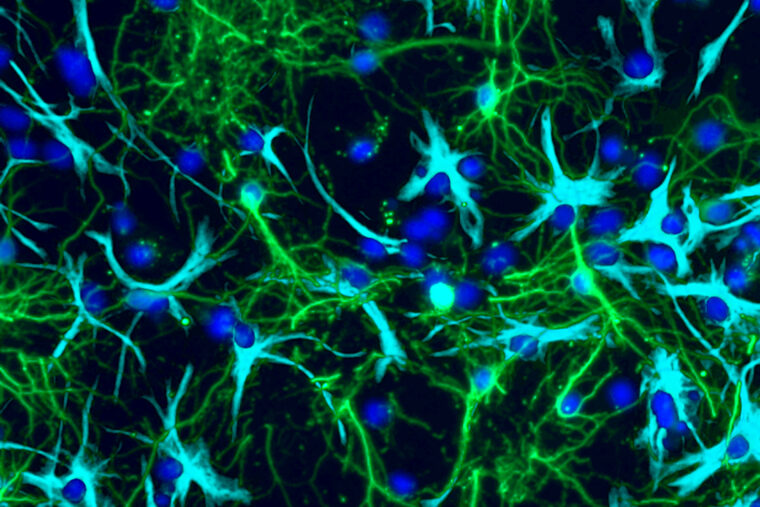
Neurons (green) and tumor cells (cyan) grown in a lab demonstrate how the two cell types can become interconnected as a tumor grows, disrupting brain development and function. New research from WashU Medicine reveals how normal electrical signals from neurons may be co-opted by certain pediatric brain tumors to boost their own growth, suggesting novel pathways for treatment. (Image: Corina Anastasaki/WashU Medicine)
<関連情報>
- https://source.washu.edu/2025/09/study-sheds-light-on-how-pediatric-brain-tumors-grow/
- https://medicine.washu.edu/news/study-sheds-light-on-how-pediatric-brain-tumors-grow/
- https://www.cell.com/neuron/fulltext/S0896-6273(25)00591-4
グルタミン酸受容体とチロシンキナーゼ受容体の異常な結合が脳腫瘍増殖の神経制御を可能にする Aberrant coupling of glutamate and tyrosine kinase receptors enables neuronal control of brain-tumor growth
Corina Anastasaki ∙ Rui Mu ∙ Chloe M. Kernan ∙ … ∙ Steven J. Mennerick ∙ Fausto J. Rodriguez ∙ David H. Gutmann
Neuron Published:September 1, 2025
DOI:https://doi.org/10.1016/j.neuron.2025.08.005
Highlights
- scRNA-seq reveals enriched glutamatergic signaling in human pediatric gliomas
- Human pediatric low-grade gliomas exhibit a glutamate growth dependency
- Glutamate stimulation enhances tumor cell ERK signaling, not membrane excitability
- Glutamate receptor activation of PDGFRα is mediated by Src to drive glioma growth
Summary
Direct and paracrine neuron-cancer interactions govern tumor development and progression. While neuron-elaborated neurotransmitters, like glutamate, support neoplastic growth, the mechanism underlying tumor intracellular mitogenic signaling and proliferation remains an unresolved question in cancer neuroscience. Herein, we discover that glutamate receptor (GluR) stimulation phosphorylates sarcoma proto-oncogene (Src) to activate platelet-derived growth factor (PDGF) receptor-α (PDGFRα)-dependent extracellular-regulated kinase (ERK) signaling and drive glioma growth. Using single-cell transcriptomic datasets and unique laboratory-generated humanized models of the most common brain tumor in children (pilocytic astrocytoma [PA]), we identify glutamatergic pathway enrichment in tumor cells, where glutamate increases PA proliferation without changing membrane depolarization. Aberrant GRID2 and GRIK3 GluR expression increases rat sarcoma (RAS)/ERK signaling by selective Src-mediated PDGFRα activation. Moreover, genetic or pharmacologic GRID2/GRIK3 and PDGFRA inhibition reduce PDGFRα/RAS/ERK activation, PA cell proliferation, and PA xenograft growth. Taken together, these observations establish a conceptual framework for understanding similar neurotransmitter dependencies in other cancers.