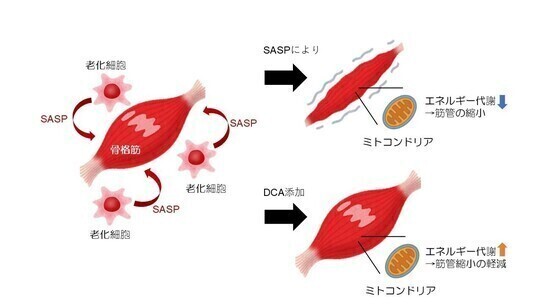
2025-09-19 東北大学
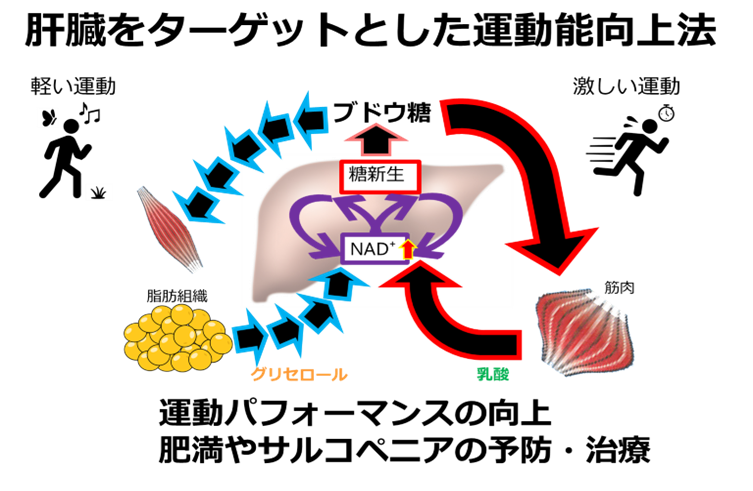
図4. 運動の強さに合わせて、グリセロールや乳酸からの糖新生の流れを促進させることは、運動能向上や肥満やサルコペニアの予防・治療につながる。
<関連情報>
- https://www.tohoku.ac.jp/japanese/2025/09/press20250919-01-Redox.html
- https://www.tohoku.ac.jp/japanese/newimg/pressimg/tohokuuniv-press20250919_01web_Redox.pdf
- https://www.nature.com/articles/s42255-025-01373-z
酸化還元依存性肝臓糖新生がマウスにおける異なる強度の運動に影響を与える Redox-dependent liver gluconeogenesis impacts different intensity exercise in mice
Takahiro Horiuchi,Keizo Kaneko,Shinichiro Hosaka,Kenji Uno,Seitaro Tomiyama,Kei Takahashi,Maya Yamato,Akira Endo,Hiroto Sugawara,Yohei Kawana,Yoichiro Asai,Shinjiro Kodama,Junta Imai,Seiya Mizuno,Satoru Takahashi,Atsushi Takasaki,Hiraku Ono,Koutaro Yokote,Rae Maeda,Yuki Sugiura & Hideki Katagiri
Nature Metabolism Published:18 September 2025
DOI:https://doi.org/10.1038/s42255-025-01373-z
Abstract
Hepatic gluconeogenesis produces glucose from various substrates to meet energy demands. However, how these substrates are preferentially used under different conditions remains unclear. Here, we show that preferential supplies of lactate and glycerol modulate hepatic gluconeogenesis, thereby impacting high-intensity and low-intensity exercise capacities, respectively. We find that liver-specific knockout of phosphoenolpyruvate carboxykinase 1 (L-Pck1KO), which blocks gluconeogenesis from lactate, decreases high-intensity exercise capacity but increases low-intensity exercise capacity by enhancing gluconeogenesis from glycerol. Conversely, liver-specific knockout of glycerol kinase (L-GykKO), which inhibits glycerol-derived gluconeogenesis, induces the opposite effects by enhancing gluconeogenesis from lactate. Given that these compensatory steps depend on NAD+-mediated oxidation in the cytosol, we hepatically expressed NADH oxidase from Lactobacillus brevis (LbNOX) to decrease the cytosolic [NADH]/[NAD+] ratio. We find that hepatic LbNOX expression enhances gluconeogenesis from both redox-dependent substrates and increases exercise capacities at both intensities. Importantly, LbNOX-induced enhancement of high-intensity and low-intensity exercise capacities is abolished in L-Pck1KO and L-GykKO mice, respectively. Therefore, supplies of gluconeogenic substrates and cytosolic redox states, rather than altered enzyme expressions, modulate hepatic gluconeogenesis and exercise capacity at different intensities. Globally, this study shows that regulating hepatic gluconeogenesis through cytosolic redox states is a potent strategy for increasing exercise performance.