2025-09-29 インペリアル・カレッジ・ロンドン(ICL)
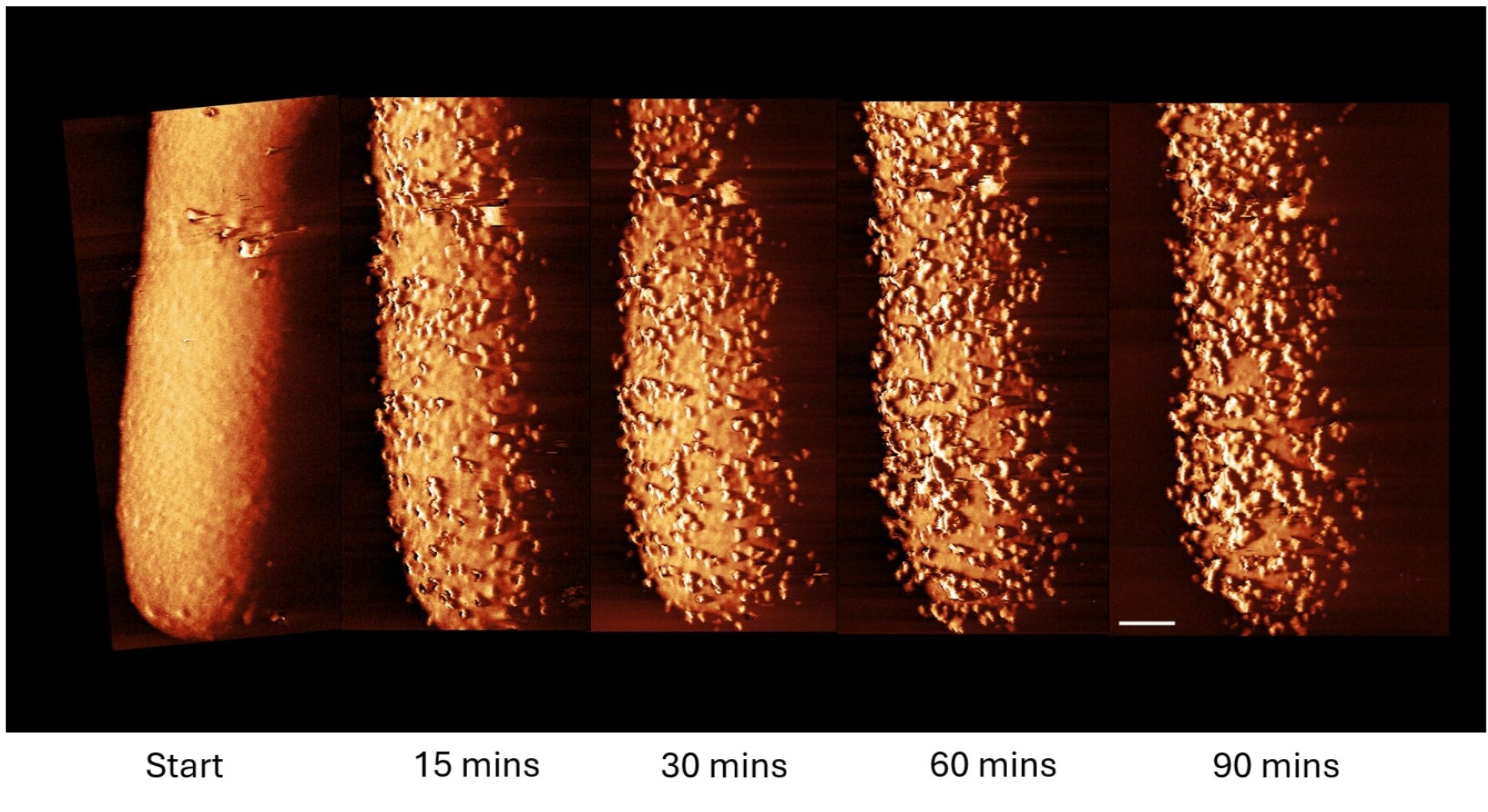 Bacteria response to antibiotics (Credit: XXX)
Bacteria response to antibiotics (Credit: XXX)
<関連情報>
- https://www.imperial.ac.uk/news/269074/amazing-images-show-antibiotics-pierce-bacterial/
- https://www.nature.com/articles/s41564-025-02133-1
ポリミキシンBの致死にはエネルギー依存的な外膜破壊が必要 Polymyxin B lethality requires energy-dependent outer membrane disruption
Carolina Borrelli,Edward J. A. Douglas,Sophia M. A. Riley,Aikaterini Ellas Lemonidi,Gerald Larrouy-Maumus,Wen-Jung Lu,Boyan B. Bonev,Andrew M. Edwards &Bart W. Hoogenboom
Nature Microbiology Published:29 September 2025
DOI:https://doi.org/10.1038/s41564-025-02133-1
Abstract
Polymyxin antibiotics target lipopolysaccharides (LPSs) in both membranes of the bacterial cell envelope, leading to bacterial killing through a poorly defined mechanism. Here we demonstrate that metabolic activity is essential for the lethality of clinically relevant doses of polymyxin B (PmB) and leverage this insight to determine its mode of action. PmB killed exponential-phase Escherichia coli but did not eliminate stationary-phase cells unless a carbon source was available. Antibiotic lethality correlated with surface protrusions visible by atomic force microscopy and LPS loss from the outer membrane via processes that required LPS synthesis and transport but that were blocked by the MCR-1 polymyxin resistance determinant. While energy-dependent outer-membrane disruption was not directly lethal, it facilitated PmB access to the inner membrane, which the antibiotic permeabilized in an energy-independent manner, leading to cell death. This work reveals how metabolic inactivity confers tolerance of an important, membrane-targeting antibiotic.