2025-10-16 東京科学大学
Web要約 の発言:
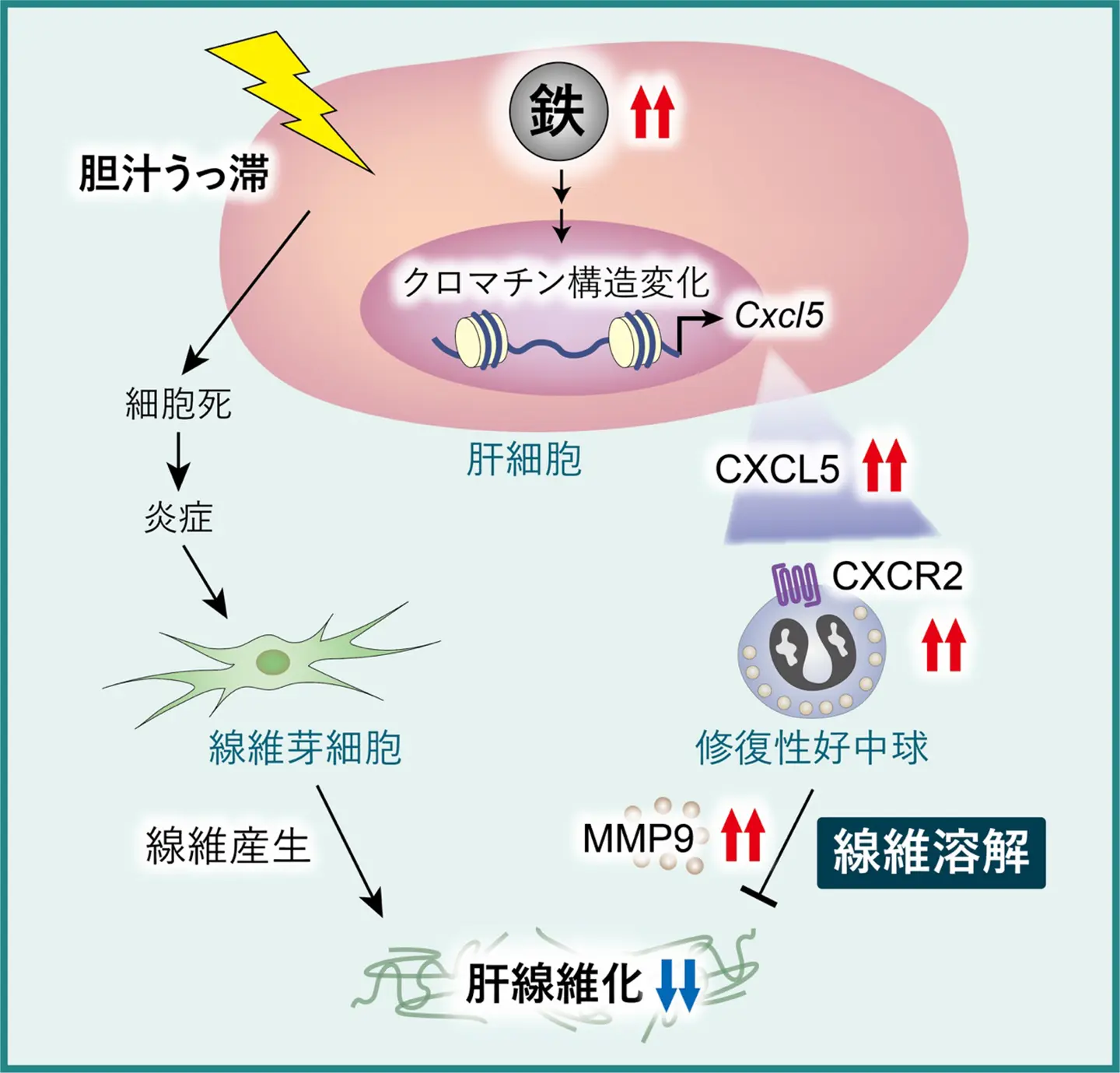
図:肝細胞内の鉄が、胆汁うっ滞性肝疾患において線維化抑制作用を発揮する仕組み
<関連情報>
- https://www.isct.ac.jp/ja/news/lzwhhtixtcus
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2422&prevId=&key=d5f5f4c70e5bed16ebeeb32c8776af51.pdf
- https://www.jhep-reports.eu/article/S2589-5559(25)00272-1/fulltext
肝細胞鉄は胆汁うっ滞における線維化性好中球動員を介して肝線維化を抑制する Hepatocyte iron suppresses liver fibrosis via fibrolytic neutrophil recruitment in cholestasis
Yohei Kanamori ∙ Akihiro Nita ∙ Keiichi I. Nakayama ∙ Daisuke Kurotaki ∙ Kenichi Harada ∙ Toshiro Moroishi
JHEP Reports Published:September 11, 2025
DOI:https://doi.org/10.1016/j.jhepr.2025.101590
Highlights
- Hepatocyte iron promotes MMP9-mediated fibrolysis, thereby suppressing liver fibrosis
- Hepatocyte iron enhances MMP9+ neutrophil influx via upregulation of CXCL5 expression
- Iron epigenetically activates the Cxcl5 promoter in hepatocytes
- MMP9 level is inversely associated with liver fibrosis in primary biliary cholangitis
Abstract
Background & Aims
Although it is well-documented that iron promotes hepatocyte death in chronic liver disease, recent studies have suggested that iron in hepatocytes also plays a protective role against chronic liver disease. However, the mechanisms underlying this beneficial role of iron remain poorly understood.
Methods
F-box and leucine-rich repeat protein 5 (FBXL5) is a substrate recognition component of the SCF E3 ligase complex that restricts intracellular iron levels. To investigate the role of hepatic iron in the pathogenesis of cholestatic liver disease, liver-specific FBXL5-deficient or control mice were fed a diet supplemented with 3,5-diethoxycarbonyl-1,4-dihydrocollidine (n = 3–12). Moreover, MMP9 expression and liver fibrosis were analyzed in liver specimens obtained from 37 patients with primary biliary cholangitis (PBC).
Results
Liver-specific FBXL5-deficient mice, which exhibits hepatic iron overload, are protected against liver fibrosis in cholestatic liver disease (Sirius red+ area, 3.1% vs. 5.3%, P = 0.005) without a reduction in fibrogenesis. The upregulation of MMP9-mediated fibrolysis accounts for resistance against liver fibrosis in these mice. Iron promotes CXCL5 expression in hepatocytes (mRNA, 2.6-fold, P = 0.001), accelerating MMP9+ neutrophil recruitment. Mechanistically, iron decreases H3K27 methylation (14% decrease, P < 0.05) and increases chromatin accessibility in the Cxcl5 promoter. Furthermore, we report an inverse association between MMP9 expression and liver fibrosis in patients with PBC (Sirius red+ area, 7.3% in MMP9high group vs. 9.7% in MMP9low group, P = 0.04).
Conclusions
Our data link hepatocyte iron with fibrolysis pathways in the setting of chronic liver disease. The present study provides insights into the pro-resolving roles of neutrophils in cholestatic liver disease.
Impact and implications
In this study, we show that hepatocyte iron suppresses liver fibrosis in cholestatic liver disease. Mechanistically, hepatocyte iron epigenetically upregulates CXCL5 expression, thereby promoting hepatic recruitment of MMP9+ fibrolytic neutrophils. Additionally, hepatic MMP9 expression is inversely associated with liver fibrosis in patients with primary biliary cholangitis. These findings reveal a protective role for hepatocyte iron and offer new therapeutic insights for chronic liver disease.