2025-11-27 浙江大学(ZJU)
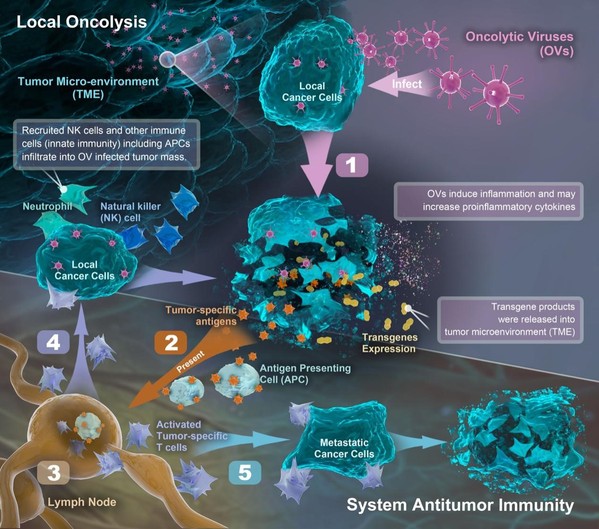
図1.腫瘍溶解性ウイルスが悪性腫瘍を殺し、腫瘍特異的免疫を強化するTMEの 作用機序を説明する模式図
<関連情報>
- https://www.zju.edu.cn/english/2025/1127/c19573a3110923/page.psp
- https://gut.bmj.com/content/early/2025/09/29/gutjnl-2025-335904
肝内胆管癌における多装甲腫瘍溶解性HSV-1(VG161)の仮説生成評価:多施設共同研究からの知見の統合 Hypothesis-generating evaluation of multi-armoured oncolytic HSV-1 (VG161) in intrahepatic cholangiocarcinoma: pooled insights from multicentre studies
Yinan Shen,Xinyan Jin,Wei Song,Tian Fang,Yuwei Li,Zeda Zhao,Xingmei Liang,Qian Tan,Ronghua Zhao,Yuntao Zhang,William Jia,,Hongwei Huang,Tengjie Wu,Guoming Shi,Zhewei Zhang,Enliang Li,Guyue Wei,Tao Jiang,Zijun Wang,Zifan Yang,Danni Lin,Linghao Xia,Sida Guo,Jiaxin Li,Fang Wei,Xueyan Shi,Siyuan Chen,Chuntian Tu,Zhanyi Shou,,Longshen Xie,Hongchao Zhang,Hangyu Zhou,Peilin Lan,Ding,Wei Chen,Yufu Ye,Xiaozhen Zhang,Yiwen Chen,Xiang Li,Zhenglong Zhai,Wendi Hu,Xiaoyu Zhang,Lei Wang,Xiaoli Sun,Qingwei Zhao,Xingjiang Hu,Youlei Wang,Zhuojun Zhou,Jiejing Kai,Jichen Li,Wei Zhang,Xueli Bai,Tingbo Liang
Gut Published: September 29, 2025
DOI:https://doi.org/10.1136/gutjnl-2025-335904
Abstract
Background Intrahepatic cholangiocarcinoma (ICC) is a highly aggressive malignancy with limited treatment options and poor prognosis, especially for patients who failed standard therapies.
Objective To explore the safety, efficacy and immunological mechanisms of the novel edition of oncolytic virus vaccination, VG161, a multiarmed oncolytic herpes simplex virus-1 expressing interleukin (IL)-12, IL-15 and a programmed death-ligand 1 antagonist, in patients with advanced ICC.
Design This pooled analysis integrates data from two multicentre clinical studies: a Phase I dose-escalation study and a Phase IIa exploratory study. 24 patients with advanced ICC received ultrasound-guided intratumoral injections of VG161. Multiomics analyses were performed on longitudinal tumour biopsies to evaluate immune modulation.
Results The oncolytic virus therapy VG161 was well tolerated and showed encouraging antitumour activity, including improved overall survival versus second-line FOLFOX chemotherapy, even though most patients received VG161 as third-line or later therapy. Notably, patients previously treated with immune checkpoint inhibitors (CPIs) experienced enhanced benefit. Multiomics profiling of longitudinal biopsies revealed significant remodelling of the immunosuppressive tumour microenvironment, with proliferated infiltration of antigen-presenting cells, CD8+ T cell activation and M2-like macrophage depletion. Single-cell and spatial transcriptomics identified epithelial and macrophage subpopulations (Epi-C2 and Macro-C1QC) as potential biomarkers of response and resistance.
Conclusion These early-phase findings suggest that VG161 elicits meaningful immune activation in ICC and supports further investigation. By inducing both direct oncolysis and multilayered immune activation, VG161 shows clinical benefit in a heavily pretreated population and holds promise for integration with CPI-based regimens. Validation in larger trials is warranted.