2025-12-03 ロイヤルメルボルン工科大学(RMIT)
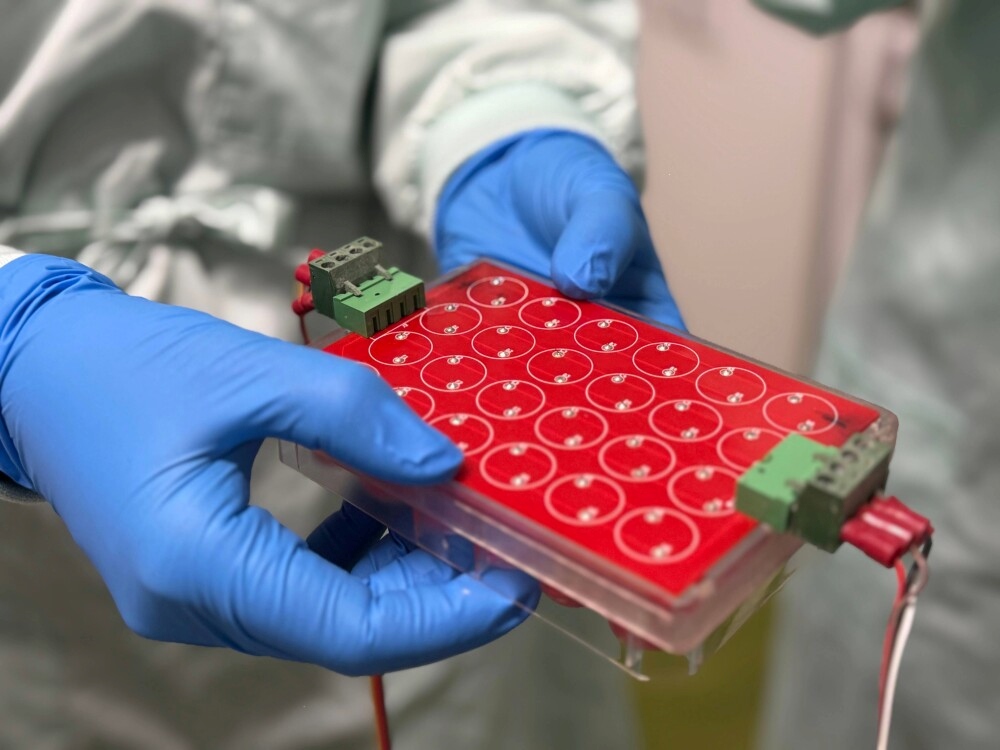
Custom-built device with electrodes for applying varied electrical signals to stem cells in culture. Credit: Will Wright, RMIT University
<関連情報>
- https://www.rmit.edu.au/news/all-news/2025/dec/zapping-stem-cells
- https://advanced.onlinelibrary.wiley.com/doi/10.1002/admi.202500403
幹細胞バイオメカニクスのライブ定量特性評価 Live Quantitative Characterization for Stem Cell Biomechanics
Kaiwen Zhang, Chayla L. Reeves, Joseph D. Berry, Kate E. Fox, Aaron Elbourne, Amy Gelmi
Advanced Materials Interfaces Published: 04 September 2025
DOI:https://doi.org/10.1002/admi.202500403
Abstract
In this study, the novel use of high-resolution force-curve imaging is demonstrated using Atomic Force Microscopy (AFM) to continuously map topographical and biomechanical changes in individual bone marrow-derived hMSCs simultaneously with chemical stimulation. The cell’s effective stiffness, quantified as Young’s modulus, is largely determined by the cytoskeletal structure within the individual cell. Chemical treatments that modulate cytoskeletal dynamics induced measurable changes in the effective stiffness, reflecting changes in cytoskeletal components such as the actin and microtubule networks. Cytochalasin D, blebbistatin, and nocodazole all reduced the effective stiffness of the cells, while colchicine increased the effective stiffness, and this is quantified across whole cells and individual actin fibers over a 200 min period. Performing these measurements continually enabled a temporal analysis of distinct locations and features across the cell with a high degree of spatial confidence.