2026-01-13 東京都医学総合研究所
<関連情報>
- https://www.igakuken.or.jp/topics/2026/0113.html
- https://link.springer.com/article/10.1186/s40478-025-02177-8
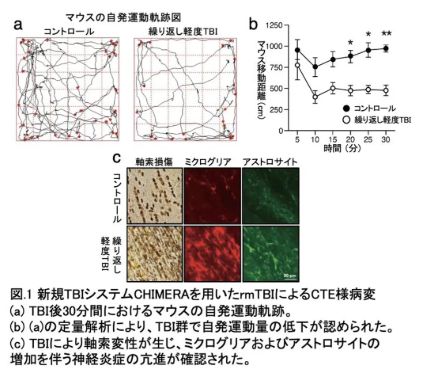
閉鎖頭部衝撃モデル(CHIMERA)を用いた反復性軽度外傷性脳損傷は、タウ遺伝子導入マウスにおけるタウ病理を促進し、タウ線維を注入した脳内でのその伝播を促進する Repetitive mild traumatic brain injury with the closed-head impact model of engineered rotational acceleration (CHIMERA) promotes tau pathology in tau transgenic mice and its propagation in brains injected with tau fibrils
Taeko Kimura,Masami Masuda-Suzukake,Masashi Hashimoto,Kazunari Sekiyama,Fuyuki Kametani,Taisuke Tomita,Hirofumi Aoyagi,Shin-ichi Hisanaga & Masato Hasegawa
Acta Neuropathologica Communications Published:08 January 2026
DOI:https://doi.org/10.1186/s40478-025-02177-8
Abstract
Chronic traumatic encephalopathy (CTE) is a progressive neurodegenerative disease characterized by the presence of abnormally phosphorylated tau aggregates. Tau is a microtubule-associated protein expressed mainly in axons of neurons with a role in the regulation of microtubule dynamics and axonal transport. It is totally unknown when and how tau is abnormally hyperphosphorylated in CTE brains. Unlike other tauopathies such as Alzheimer’s disease, in which diseases start several decades before clinical symptoms and the time point of onset is not clear, in the case of CTE it is evident when and what impacts are given to cause the diseases. Repetitive mild traumatic brain injury (rmTBI) is a known causative factor for CTE, particularly in athletes engaged in contact sports, individuals involved in traffic accidents, and military personnel exposed to blast injuries. We hypothesized rmTBI to be a useful experimental paradigm for investigating the initial processes of tau hyperphosphorylation in CTE. Among the various experimental models for TBI reported to date, we focus on the Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA), because it appears to replicate human TBI more faithfully than other models particularly regarding on the impact mechanism and pathology. After verifying that CHIMERA rmTBI induced brain injuries analogous to human CTE, we investigated tau pathology in wild-type (WT) and P301S human tau transgenic (Tg) mice subjected to CHIMERA rmTBI. While no hyperphosphorylated tau signal was observed in any region of the WT mouse brain, an increased number of AT8-positive cells were detected in the motor and sensory cortices of P301S Tg mouse brains, accompanied by dendritic abnormalities, after rmTBI. Further, we found that CHIMERA rmTBI enhanced the spreading of tau pathology in brains of WT mice when tau fibrils were inoculated. These results suggest a possibility that rmTBI constitutes a risk factor stimulating the progression and propagation of tau pathology, rather than causing the initial events, if tau aggregates are present in the brain and that CHIMERA rmTBI may serve as a valuable experimental model for investigating its molecular mechanisms.


