2026-01-09 中国科学院(CAS)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202601/t20260112_1145606.shtml
- https://www.nature.com/articles/s41589-025-02123-8
脱アセチル化酵素非依存性HDAC1凝縮が神経膠芽腫におけるテモゾロミド反応を規定する Deacetylase-independent HDAC1 condensation defines temozolomide response in glioblastoma
Qinkai Zhang,Ru Qiu,Bing Lu,Jinhong Wang,Jizhao Cao,Hongni Zhu,Meng Huang,Wenyong Long,Ke Fang,Chuanxia Zhang,Fuxi Li,Wei Shi,Qing Liu,Yiming Li,Peng Dong & Wei Zhao
Nature Chemical Biology Published:09 January 2026
DOI:https://doi.org/10.1038/s41589-025-02123-8
Abstract
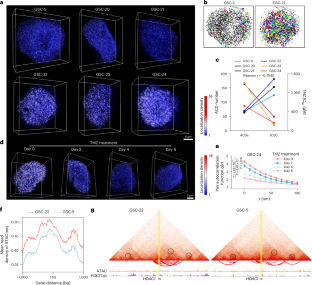
Temozolomide is a standard-of-care therapeutic agent for glioblastoma. However, persons who initially respond well often experience a notable reduction in efficacy over time, with the underlying mechanisms remaining unclear. Here we demonstrate that the reduced response to temozolomide correlates with decreased chromatin accessibility, marked by reduced H3K27ac modification and alterations in chromatin loops. Mechanistically, temozolomide treatment upregulates histone deacetylase 1 (HDAC1) expression. Intriguingly, increased HDAC1 forms condensates independently of its deacetylase function. These condensates arise from multivalent interactions within the intrinsically disordered region and specific interactions with CCCTC-binding factor (CTCF), facilitating resistance to temozolomide by promoting the assembly of DNA repair complexes, even in the absence of direct deacetylase activity of HDAC1. Through phase-separation-based screening, we identified resminostat as an effective disruptor of HDAC1–CTCF condensates, thereby restoring temozolomide sensitivity in patient-derived xenograft models. Our findings introduce deacetylase-independent HDAC1 condensation as a distinct mechanism regulating temozolomide response, providing valuable insights into potential therapeutic strategies.


