2026-01-23 東京科学大学
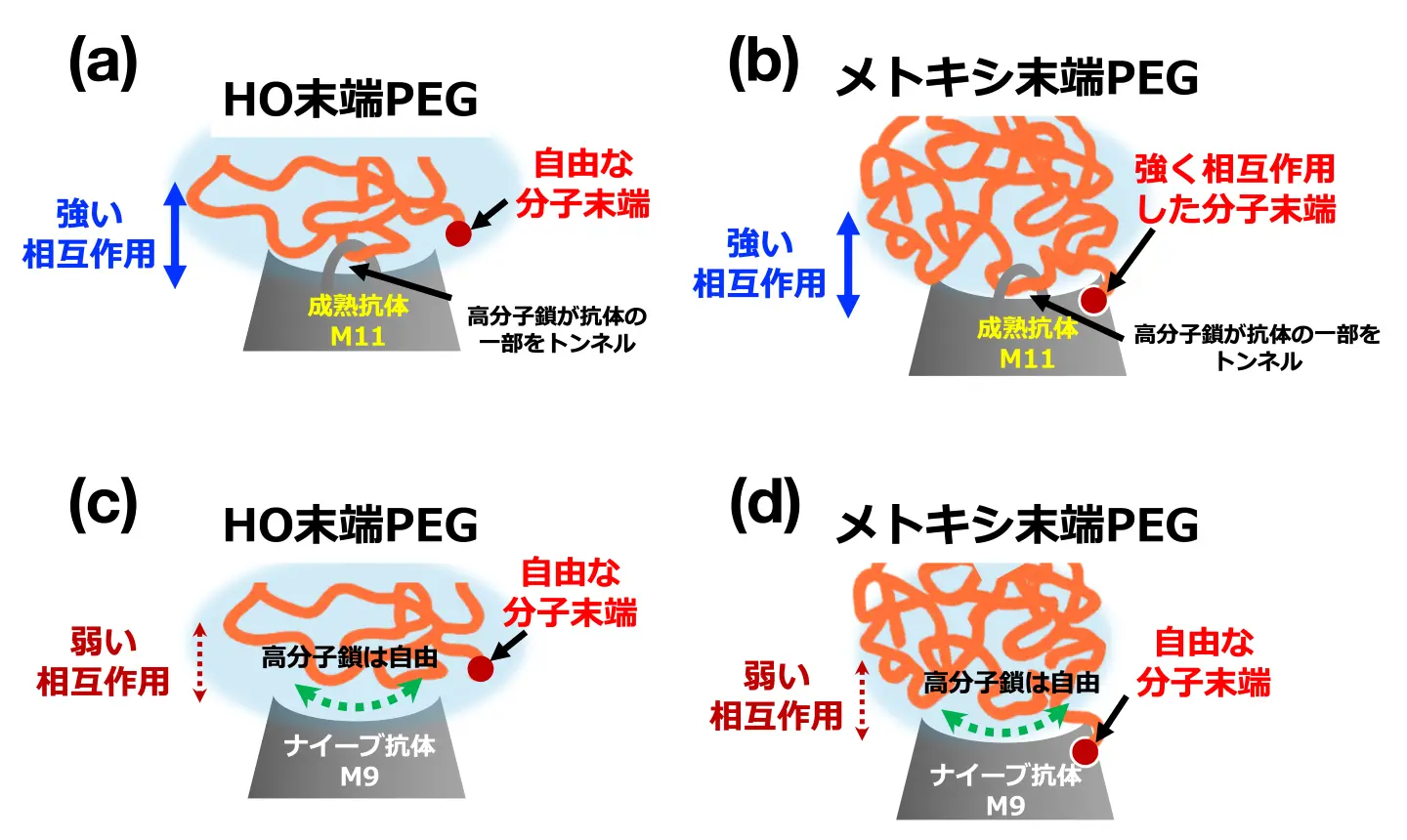
図1. ナイーブ・成熟抗体と末端基の異なるPEG鎖の相互作用の模式図
<関連情報>
- https://www.isct.ac.jp/ja/news/5x0y59vrf46h
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2969&prevId=&key=6ae67412ec55b8f2923fa7b8c846fa4e.pdf
- https://pubs.acs.org/doi/10.1021/acsabm.5c01967
PEG-抗PEG抗体相互作用のAFMベース単分子力分光法 AFM-Based Single-Molecule Force Spectroscopy of PEG–Anti-PEG Antibody Interactions
Glenn Villena Latag,Hiroyuki Tahara,Airi Katase,Shoichi Maeda,Yiwei Liu,Yoshimitsu Kakuta,Takamasa Teramoto,Takeshi Mori,and Tomohiro Hayashi
ACS Applied Bio Materials Published January: 20, 2026
DOI:https://doi.org/10.1021/acsabm.5c01967
Abstract
Poly(ethylene glycol) (PEG) is widely used as a stealth polymer to enhance drug stability and circulation by reducing immune recognition. However, anti-PEG antibodies are increasingly reported in humans, leading to accelerated drug clearance and adverse immune responses. While ensemble assays have clarified the scheme of PEG–antibody binding, they lack the resolution to probe molecular-scale mechanics. Here, we used atomic force microscopy-based single-molecule force spectroscopy (AFM-SMFS) to examine how PEG terminal chemistry and antibody maturation modulate these interactions. Methoxy- (m-PEG) and hydroxy-terminated PEG (HO-PEG) were tested against Fv-clasps from two anti-PEG IgMs: the naïve IgM M9 and the affinity-matured IgM M11. M11 bound PEG more strongly and at shorter rupture distances than M9, with 2D force–distance maps revealing the most intense signatures for M11 and m-PEG pair. Complementary quartz crystal microbalance with dissipation (QCM-D) and Fourier-transform infrared (FTIR) spectroscopy confirmed higher binding by M11 and a terminal preference of M9 for m-PEG. In addition to antibody maturation, we report that the hydrated structure of PEG plays a significant role in PEG–antibody binding. HO-PEG forms extended, hydrated layers, whereas m-PEG adopts compact, collapsed conformations, shaping antibody accessibility and binding mechanics. These results provide molecular-level insight into how antibody structure and PEG hydration state dictate binding, offering design principles for PEGylated therapeutics with reduced immunogenicity and improved performance.


