2026-02-19 リンショーピング大学
<関連情報>
- https://liu.se/en/news-item/treatment-can-protect-extremely-premature-babies-from-lung-disease
- https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2845265
超早産児における早期予防的ヒドロコルチゾン投与と気管支肺異形成症フリー生存率 Early Prophylactic Hydrocortisone and Bronchopulmonary Dysplasia–Free Survival in Extremely Preterm Infants
Veronica Smedbäck, MS; Lars J. Björklund, MD, PhD; Anders Flisberg, MD, PhD;et al
JAMA Network Open Published:February 19, 2026
DOI:10.1001/jamanetworkopen.2025.60146

Key Points
Question After guideline implementation, has early prophylactic hydrocortisone improved survival without bronchopulmonary dysplasia (BPD) in extremely preterm infants born in Sweden, and is it safe to use?
Findings In this cohort study using prospectively collected data from 1106 infants from a national register, introduction of prophylactic hydrocortisone was followed by an increased likelihood of survival without BPD. There was no significant increase in severe neonatal morbidities.
Meaning These findings, based on clinical implementation data, suggest alignment with previous similar studies supporting the benefits and safety of early prophylactic hydrocortisone treatment.
Abstract
Importance In randomized trials, early prophylactic hydrocortisone improved survival without bronchopulmonary dysplasia (BPD) with few adverse effects in extremely preterm infants. Large scale implementation data are needed to evaluate clinical effects and safety.
Objective To examine the association between early prophylactic hydrocortisone and survival without BPD at 36 weeks’ postmenstrual age (PMA) in extremely preterm infants in Sweden after guideline implementation and to assess treatment safety.
Design, Setting, and Participants A national historical cohort study with prospectively collected data from the Swedish Neonatal Quality register from 4 Swedish centers where hydrocortisone prophylaxis was implemented. The study included infants born between 22 and 27 weeks’ gestation between 2018 and 2023. Infants were divided into exposed and nonexposed groups according to the intention-to-treat principle.
Exposure Hydrocortisone, 1 mg/kg/d, for the first 7 days of life, followed by 0.5 mg/kg/d from days 8 through 10.
Main outcomes and measures The primary outcome was survival without BPD at 36 weeks’ PMA. A predefined statistical analysis plan with logistic regression was used to calculate unadjusted and adjusted odds ratios.
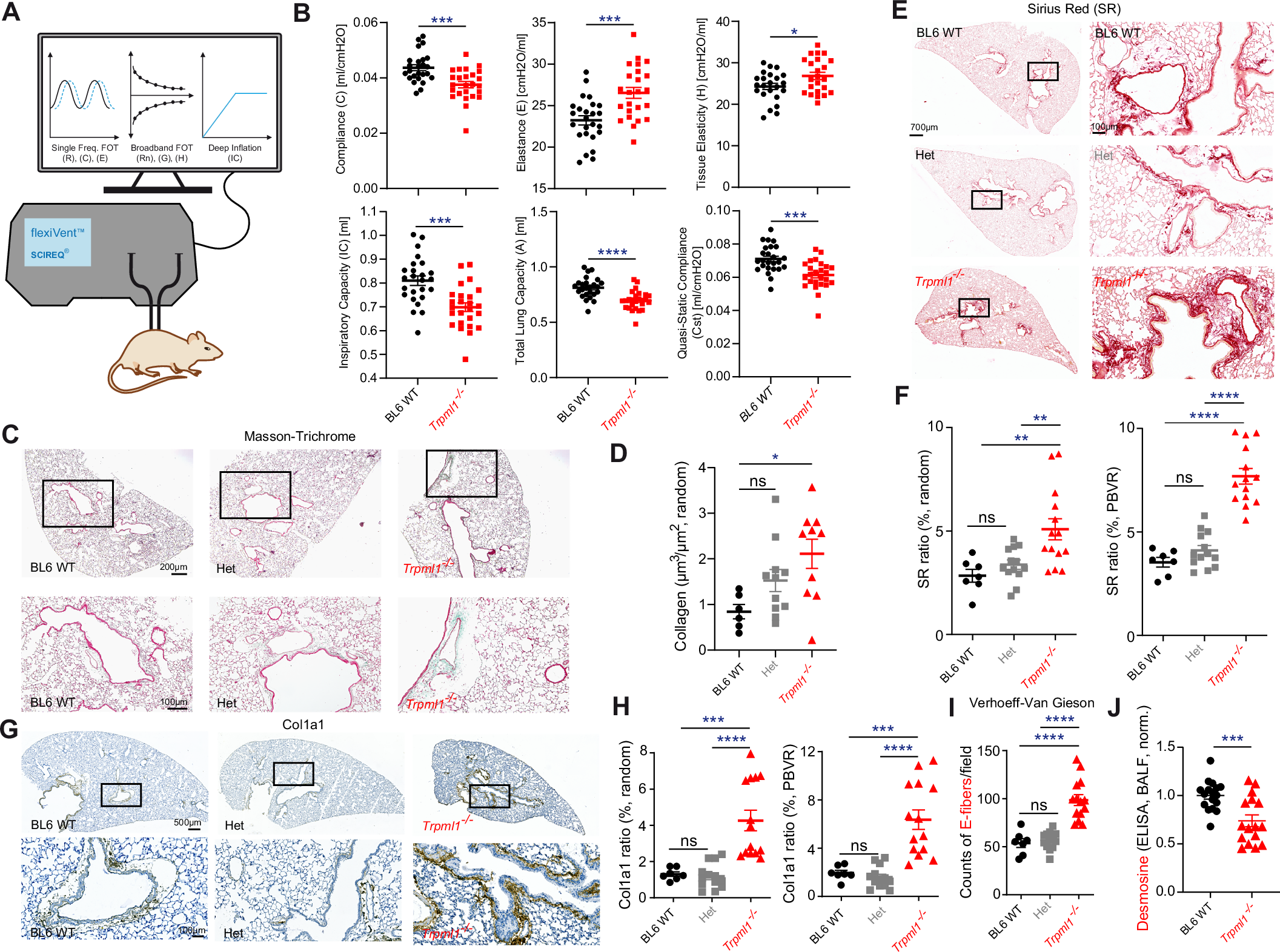
Results Among 1106 infants (median [IQR] gestational age, 25 weeks, 6 days [24 weeks, 3 days to 27 weeks]; median [IQR] birth weight, 780 [610-964] g), 474 received hydrocortisone prophylaxis and 632 did not. Survival without BPD occurred in 154 of 474 exposed (32.5%) and 185 of 632 nonexposed (29.3%) infants (adjusted odds ratio, 1.62; 95% CI, 1.16-2.27). BPD occurred in 233 exposed (49.2%) and 345 nonexposed (54.6%) infants (adjusted odds ratio, 0.65; 95% CI, 0.49-0.86). Death before 36 weeks’ PMA occurred in 87 exposed (18.4%) and 102 nonexposed (16.1%) infants. Late-onset bacterial infection was more common in exposed infants, but not significant after adjustment. No other severe neonatal morbidities differed significantly between the 2 groups.
Conclusions and relevance In this cohort study of extremely preterm infants, the introduction of prophylactic hydrocortisone was associated with increased survival without BPD, after adjusting for covariates. There was no significant increase in severe neonatal morbidities, except that late-onset bacterial infection was more common in the exposed group before adjustments.