H1N1タンパク質のダイナミックな動きから、新たな脆弱性が判明 Dynamic movement of H1N1 proteins reveal new vulnerabilities
2023-01-25 カリフォルニア大学サンディエゴ校(UCSD)
◆インフルエンザ・ワクチンの主な標的は、ヘマグルチニン(HA)とノイラミニダーゼ(NA)という2つの表面糖タンパク質です。HAタンパク質はウイルスが宿主細胞に結合するのを助ける一方、NAタンパク質はハサミのように作用してHAを細胞膜から切り離し、ウイルスの複製を可能にする。両糖タンパク質の特性は以前から研究されていたが、その動きを完全に理解することはできなかった。
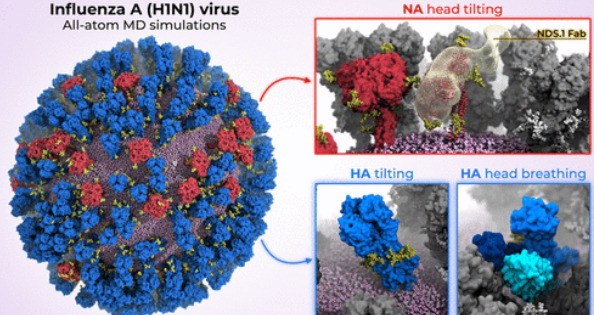
◆カリフォルニア大学サンディエゴ校の研究者らは、H1N1ウイルスの原子レベルのコンピューターモデルを初めて作成し、糖タンパク質の「呼吸」と「傾き」の動きから新たな脆弱性を明らかにした。この研究は、ACS Central Science誌に掲載され、将来のインフルエンザワクチンや抗ウイルス剤の設計に役立つ可能性を示唆しています。
<関連情報>
- https://today.ucsd.edu/story/computer-model-influenza
- https://pubs.acs.org/doi/full/10.1021/acscentsci.2c00981
呼吸と傾き:メソスケールシミュレーションが明らかにしたインフルエンザ糖タンパク質の脆弱性 Breathing and Tilting: Mesoscale Simulations Illuminate Influenza Glycoprotein Vulnerabilities
Lorenzo Casalino, Christian Seitz, Julia Lederhofer, Yaroslav Tsybovsky, Ian A. Wilson, Masaru Kanekiyo, and Rommie E. Amaro
ACS Central Science Published:December 8, 2022
DOI:https://doi.org/10.1021/acscentsci.2c00981

Abstract
Influenza virus has resurfaced recently from inactivity during the early stages of the COVID-19 pandemic, raising serious concerns about the nature and magnitude of future epidemics. The main antigenic targets of influenza virus are two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Whereas the structural and dynamical properties of both glycoproteins have been studied previously, the understanding of their plasticity in the whole-virion context is fragmented. Here, we investigate the dynamics of influenza glycoproteins in a crowded protein environment through mesoscale all-atom molecular dynamics simulations of two evolutionary-linked glycosylated influenza A whole-virion models. Our simulations reveal and kinetically characterize three main molecular motions of influenza glycoproteins: NA head tilting, HA ectodomain tilting, and HA head breathing. The flexibility of HA and NA highlights antigenically relevant conformational states, as well as facilitates the characterization of a novel monoclonal antibody, derived from convalescent human donor, that binds to the underside of the NA head. Our work provides previously unappreciated views on the dynamics of HA and NA, advancing the understanding of their interplay and suggesting possible strategies for the design of future vaccines and antivirals against influenza.