2023-03-28 オークリッジ国立研究所(ORNL)
研究は、COVID-19研究においてよりあまり研究されていない酵素であるPLproを標的とし、共有結合阻害剤と呼ばれる分子が、繁殖を助けるウイルスの増殖を制限し、宿主の免疫反応を阻害することができるとした。
共有結合を形成することで、ターゲットのタンパク質に強力に作用し、抗ウイルス治療に効果的であるとされる。
研究者は、現在も第2世代の共有結合PLpro阻害剤の開発に取り組んでおり、将来的には別のウイルスの治療薬にも利用できる可能性があるとしている。
<関連情報>
- https://www.ornl.gov/news/ornl-led-team-designs-molecule-disrupt-sars-cov-2-infection
- https://www.nature.com/articles/s41467-023-37254-w
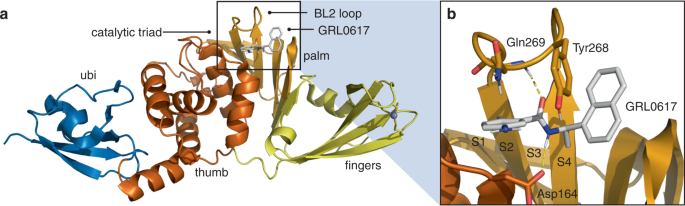
SARS-CoV-2由来パパイン様プロテアーゼの強力かつ選択的な共有結合による阻害作用 Potent and selective covalent inhibition of the papain-like protease from SARS-CoV-2
Brian C. Sanders,Suman Pokhrel,Audrey D. Labbe,Irimpan I. Mathews,Connor J. Cooper,Russell B. Davidson,Gwyndalyn Phillips,Kevin L. Weiss,Qiu Zhang,Hugh O’Neill,Manat Kaur,Jurgen G. Schmidt,Walter Reichard,Surekha Surendranathan,Jyothi Parvathareddy,Lexi Phillips,Christopher Rainville,David E. Sterner,Desigan Kumaran,Babak Andi,Gyorgy Babnigg,Nigel W. Moriarty,Paul D. Adams,Andrzej Joachimiak,Brett L. Hurst,Suresh Kumar,Tauseef R. Butt,Colleen B. Jonsson,Lori Ferrins,Soichi Wakatsuki,Stephanie Galanie,Martha S. Head & Jerry M. Parks
Nature Communications Published:28 March 2023
DOI:https://doi.org/10.1038/s41467-023-37254-w
Abstract
Direct-acting antivirals are needed to combat coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). The papain-like protease (PLpro) domain of Nsp3 from SARS-CoV-2 is essential for viral replication. In addition, PLpro dysregulates the host immune response by cleaving ubiquitin and interferon-stimulated gene 15 protein from host proteins. As a result, PLpro is a promising target for inhibition by small-molecule therapeutics. Here we design a series of covalent inhibitors by introducing a peptidomimetic linker and reactive electrophile onto analogs of the noncovalent PLpro inhibitor GRL0617. The most potent compound inhibits PLpro with kinact/KI = 9,600 M-1 s-1, achieves sub-μM EC50 values against three SARS-CoV-2 variants in mammalian cell lines, and does not inhibit a panel of human deubiquitinases (DUBs) at >30 μM concentrations of inhibitor. An X-ray co-crystal structure of the compound bound to PLpro validates our design strategy and establishes the molecular basis for covalent inhibition and selectivity against structurally similar human DUBs. These findings present an opportunity for further development of covalent PLpro inhibitors.