2023-04-17 ワシントン大学セントルイス校
治療の一つとして、患者自身の幹細胞を採取し、抗がん剤治療後に再移植するが、十分な幹細胞を採取できない場合があり、治療成績に悪影響を与えることがある。臨床試験によると、この薬剤を標準的な治療法に組み合わせることで、十分な数の幹細胞を収集できるようになった。幹細胞の回収に成功した患者は、標準治療と比較して数倍の幹細胞を採取することができた。この薬剤によって収集された幹細胞は、患者の回復を助けることができる。
<関連情報>
- https://source.wustl.edu/2023/04/investigational-drug-may-improve-stem-cell-transplantation-for-multiple-myeloma-patients/
- https://medicine.wustl.edu/news/investigational-drug-may-improve-stem-cell-transplantation-for-multiple-myeloma-patients/
- https://www.nature.com/articles/s41591-023-02273-z
- https://pubmed.ncbi.nlm.nih.gov/36779841/
多発性骨髄腫における自家移植のための造血幹細胞動員を目的としたMotixafortideとG-CSFの併用:無作為化第3相試験 Motixafortide and G-CSF to mobilize hematopoietic stem cells for autologous transplantation in multiple myeloma: a randomized phase 3 trial
Zachary D. Crees,Michael P. Rettig,Reyka G. Jayasinghe,Keith Stockerl-Goldstein,Sarah M. Larson,Illes Arpad,Giulio A. Milone,Massimo Martino,Patrick Stiff,Douglas Sborov,Denise Pereira,Ivana Micallef,Gemma Moreno-Jiménez,Gabor Mikala,Maria Liz Paciello Coronel,Udo Holtick,John Hiemenz,Muzaffar H. Qazilbash,Nancy Hardy,Tahir Latif,Irene García-Cadenas,Abi Vainstein-Haras,Ella Sorani,Irit Gliko-Kabir,Inbal Goldstein,Debby Ickowicz,Liron Shemesh-Darvish,Shaul Kadosh,Feng Gao,Mark A. Schroeder,Ravi Vij & John F. DiPersio
Nature Medicine Published:17 April 2023
DOI:https://doi.org/10.1038/s41591-023-02273-z
Abstract
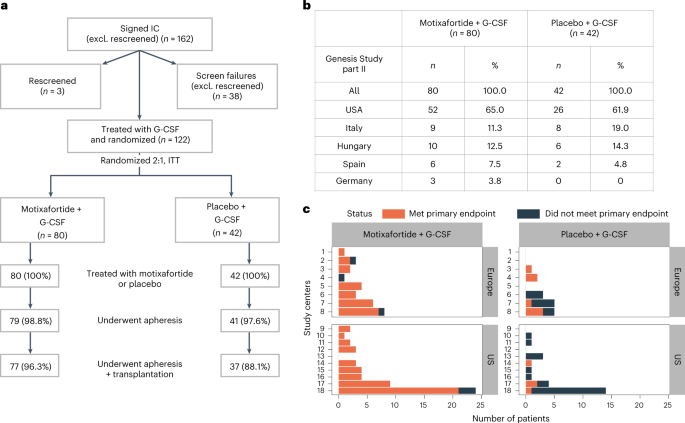
Autologous hematopoietic stem cell transplantation (ASCT) improves survival in multiple myeloma (MM). However, many individuals are unable to collect optimal CD34+ hematopoietic stem and progenitor cell (HSPC) numbers with granulocyte colony-stimulating factor (G-CSF) mobilization. Motixafortide is a novel cyclic-peptide CXCR4 inhibitor with extended in vivo activity. The GENESIS trial was a prospective, phase 3, double-blind, placebo-controlled, multicenter study with the objective of assessing the superiority of motixafortide + G-CSF over placebo + G-CSF to mobilize HSPCs for ASCT in MM. The primary endpoint was the proportion of patients collecting ≥6 × 106 CD34+ cells kg–1 within two apheresis procedures; the secondary endpoint was to achieve this goal in one apheresis. A total of 122 adult patients with MM undergoing ASCT were enrolled at 18 sites across five countries and randomized (2:1) to motixafortide + G-CSF or placebo + G-CSF for HSPC mobilization. Motixafortide + G-CSF enabled 92.5% to successfully meet the primary endpoint versus 26.2% with placebo + G-CSF (odds ratio (OR) 53.3, 95% confidence interval (CI) 14.12–201.33, P < 0.0001). Motixafortide + G-CSF also enabled 88.8% to meet the secondary endpoint versus 9.5% with placebo + G-CSF (OR 118.0, 95% CI 25.36–549.35, P < 0.0001). Motixafortide + G-CSF was safe and well tolerated, with the most common treatment-emergent adverse events observed being transient, grade 1/2 injection site reactions (pain, 50%; erythema, 27.5%; pruritis, 21.3%). In conclusion, motixafortide + G-CSF mobilized significantly greater CD34+ HSPC numbers within two apheresis procedures versus placebo + G-CSF while preferentially mobilizing increased numbers of immunophenotypically and transcriptionally primitive HSPCs. Trial Registration: ClinicalTrials.gov, NCT03246529
多発性骨髄腫の治療標的のシングルセルディスカバリーおよびマルチオームキャラクタリゼーション Single-Cell Discovery and Multiomic Characterization of Therapeutic Targets in Multiple Myeloma
Lijun Yao, Julia T Wang, Reyka G Jayasinghe, Julie O’Neal, Chia-Feng Tsai, Michael P Rettig, Yizhe Song, Ruiyang Liu, Yanyan Zhao, Omar M Ibrahim, Mark A Fiala, Julie M Fortier, Siqi Chen, Leah Gehrs, Fernanda Martins Rodrigues, Michael C Wendl, Daniel Kohnen, Andrew Shinkle, Song Cao, Steven M Foltz, Daniel Cui Zhou, Erik Storrs, Matthew A Wyczalkowski, Smrithi Mani, Scott R Goldsmith, Ying Zhu, Mark Hamilton, Tao Liu, Feng Chen, Ravi Vij, Li Ding, John F DiPersio
Cancer Research Published:2023 Apr 14
DOI:https://doi.org/10.1158/0008-5472.can-22-1769
Abstract
Multiple myeloma (MM) is a highly refractory hematologic cancer. Targeted immunotherapy has shown promise in MM but remains hindered by the challenge of identifying specific yet broadly representative tumor markers. We analyzed 53 bone marrow (BM) aspirates from 41 MM patients using an unbiased, high-throughput pipeline for therapeutic target discovery via single-cell transcriptomic profiling, yielding 38 MM marker genes encoding cell-surface proteins and 15 encoding intracellular proteins. Of these, 20 candidate genes were highlighted that are not yet under clinical study, 11 of which were previously uncharacterized as therapeutic targets. The findings were cross-validated using bulk RNA sequencing, flow cytometry, and proteomic mass spectrometry of MM cell lines and patient BM, demonstrating high overall concordance across data types. Independent discovery using bulk RNA sequencing reiterated top candidates, further affirming the ability of single-cell transcriptomics to accurately capture marker expression despite limitations in sample size or sequencing depth. Target dynamics and heterogeneity were further examined using both transcriptomic and immuno-imaging methods. In summary, this study presents a robust and broadly applicable strategy for identifying tumor markers to better inform the development of targeted cancer therapy.
Significance: Single-cell transcriptomic profiling and multiomic cross-validation to uncover therapeutic targets identifies 38 myeloma marker genes, including 11 transcribing surface proteins with previously uncharacterized potential for targeted antitumor therapy.
©2023 The Authors; Published by the American Association for Cancer Research.

