2023-05-03 カリフォルニア大学校アーバイン校(UCI)
◆研究成果は、腎不全に蓄積する腸由来の細菌依存性毒素と尿素の混合物が、血管損傷や脳の微小出血を引き起こすことを示した。CKDは、脳血管疾患のリスク要因として認識されるようになっているが、その脳への影響についてはまだ十分に理解されていない。
◆この研究は、CKDによる脳損傷の基礎的メカニズムについての重要な洞察を提供し、腎臓病の治療を含む新しい治療ターゲットを提供する。
<関連情報>
- https://news.uci.edu/2023/05/03/uc-irvine-led-study-reveals-first-clear-link-between-chronic-kidney-disease-and-stroke-risk/
- https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-023-02703-2
慢性腎臓病は脳内微小出血の形成を促進する Chronic kidney disease promotes cerebral microhemorrhage formation
Chuo Fang,Wei Ling Lau,Jiahong Sun,Rudy Chang,Adrian Vallejo,Donghy Lee,Jihua Liu,Han Liu,Yu-Han Hung,Yitong Zhao,Annlia Paganini-Hill,Rachita K. Sumbria,David H. Cribbs & Mark Fisher
Journal of Neuroinflammation Published:25 February 2023
DOI:https://doi.org/10.1186/s12974-023-02703-2
Abstract
Background
Chronic kidney disease (CKD) is increasingly recognized as a stroke risk factor, but its exact relationship with cerebrovascular disease is not well-understood. We investigated the development of cerebral small vessel disease using in vivo and in vitro models of CKD.
Methods
CKD was produced in aged C57BL/6J mice using an adenine-induced tubulointerstitial nephritis model. We analyzed brain histology using Prussian blue staining to examine formation of cerebral microhemorrhage (CMH), the hemorrhagic component of small vessel disease and the neuropathological substrate of MRI-demonstrable cerebral microbleeds. In cell culture studies, we examined effects of serum from healthy or CKD patients and gut-derived uremic toxins on brain microvascular endothelial barrier.
Results
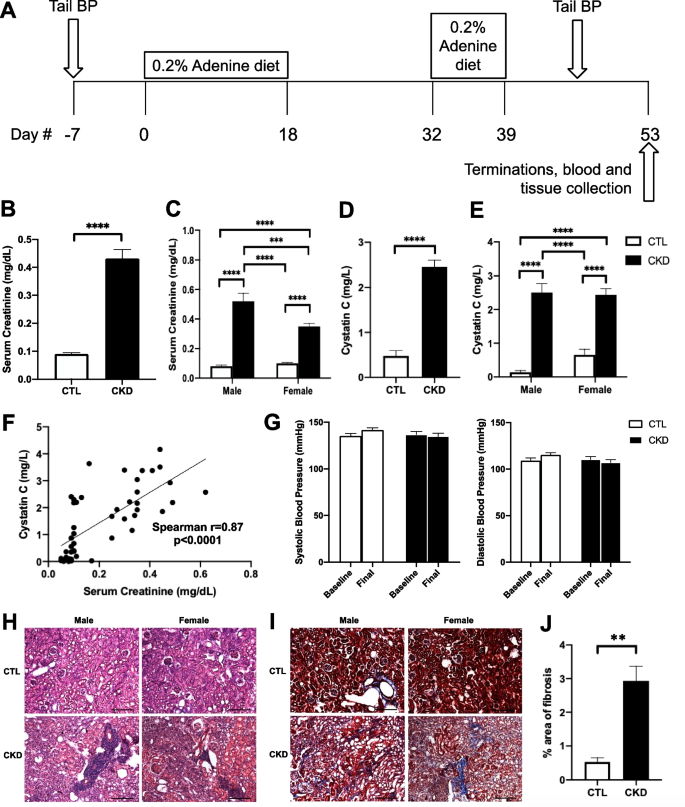
CKD was induced in aged C57BL/6J mice with significant increases in both serum creatinine and cystatin C levels (p < 0.0001) without elevation of systolic or diastolic blood pressure. CMH was significantly increased and positively correlated with serum creatinine level (Spearman r = 0.37, p < 0.01). Moreover, CKD significantly increased Iba-1-positive immunoreactivity by 51% (p < 0.001), induced a phenotypic switch from resting to activated microglia, and enhanced fibrinogen extravasation across the blood–brain barrier (BBB) by 34% (p < 0.05). On analysis stratified by sex, the increase in CMH number was more pronounced in male mice and this correlated with greater creatinine elevation in male compared with female mice. Microglial depletion with PLX3397 diet significantly decreased CMH formation in CKD mice without affecting serum creatinine levels. Incubation of CKD serum significantly reduced transendothelial electrical resistance (TEER) (p < 0.01) and increased sodium fluorescein permeability (p < 0.05) across the endothelial monolayer. Uremic toxins (i.e., indoxyl sulfate, p-cresyl sulfate, and trimethylamine-N-oxide) in combination with urea and lipopolysaccharide induced a marked drop in TEER compared with the control group (p < 0.0001).
Conclusions
CKD promotes the development of CMH in aged mice independent of blood pressure but directly proportional to the degree of renal impairment. These effects of CKD are likely mediated in part by microglia and are associated with BBB impairment. The latter is likely related to gut-derived bacteria-dependent toxins classically associated with CKD. Overall, these findings demonstrate an important role of CKD in the development of cerebral small vessel disease.


