2023-11-14 パデュー大学
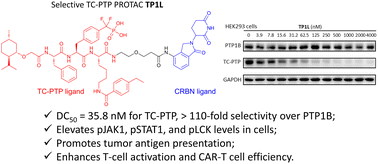
◆TC-PTPを削除することで、特定の腫瘍細胞で抗原提示が促進され、免疫系に腫瘍細胞の存在が知らされます。同時に、T細胞内で酵素の喪失はT細胞の活性化と増殖を刺激し、免疫細胞が腫瘍細胞と戦って破壊する方向に導きます。
◆これにより、個々の患者の遺伝子プロファイルに基づいた個別化された治療が可能になり、従来の免疫療法に比べて効果的かつ副作用が少ない治療法の開発が期待されています。
<関連情報>
- https://www.purdue.edu/newsroom/releases/2023/Q4/purdue-pharmaceutical-compound-sounds-the-alarm-on-cancer-cells-and-unleashes-t-cells.html
- https://pubs.rsc.org/en/Content/ArticleLanding/2023/SC/D3SC04541B
がん免疫療法のための選択的TC-PTP分解剤を発見 Discovery of a selective TC-PTP degrader for cancer immunotherapy
Jinmin Miao,Jiajun Dong,Yiming Miao,Yunpeng Bai, Zihan Qu,Brenson A. Jassim,Bo Huang,Quyen Nguyen,Yuan Ma,Allison A. Murray,Jinyue Li,Philip S. Low and Zhong-Yin Zhang
Chemical Science Published:24 Oct 2023
DOI:https://doi.org/10.1039/D3SC04541B
Abstract
T-cell protein tyrosine phosphatase (TC-PTP), encoded by PTPN2, has emerged as a promising target for cancer immunotherapy. TC-PTP deletion in B16 melanoma cells promotes tumor cell antigen presentation, while loss of TC-PTP in T-cells enhances T-cell receptor (TCR) signaling and stimulates cell proliferation and activation. Therefore, there is keen interest in developing TC-PTP inhibitors as novel immunotherapeutic agents. Through rational design and systematic screening, we discovered the first highly potent and selective TC-PTP PROTAC degrader, TP1L, which induces degradation of TC-PTP in multiple cell lines with low nanomolar DC50s and >110-fold selectivity over the closely related PTP1B. TP1L elevates the phosphorylation level of TC-PTP substrates including pSTAT1 and pJAK1, while pJAK2, the substrate of PTP1B, is unaffected by the TC-PTP degrader. TP1L also intensifies interferon gamma (IFN-γ) signaling and increases MHC-I expression. In Jurkat cells, TP1L activates TCR signaling through increased phosphorylation of LCK. Furthermore, in a CAR-T cell and KB tumor cell co-culture model, TP1L enhances CAR-T cell mediated tumor killing efficacy through activation of the CAR-T cells. Thus, we surmise that TP1L not only provides a unique opportunity for in-depth interrogation of TC-PTP biology but also serves as an excellent starting point for the development of novel immunotherapeutic agents targeting TC-PTP.