2023-12-04 パデュー大学
◆研究では、DDX5の増加がソラフェニブの効果を強化し、がん細胞の成長を抑制することが実験的に確認された。新しい治療法として、DDX5 mRNAの供給によりがん細胞を標的にし、ソラフェニブの投与期間中のみ効果を発揮するアプローチが提案されている。
<関連情報>
- https://www.purdue.edu/newsroom/releases/2023/Q4/discovery-points-to-new-approach-to-treating-liver-cancer.html
- https://link.springer.com/article/10.1038/s41419-023-06302-0
RNAヘリカーゼDDX5はWnt/β-カテニン-フェロプトーシス軸を介して肝細胞癌のソラフェニブ感受性を調節する RNA helicase DDX5 modulates sorafenib sensitivity in hepatocellular carcinoma via the Wnt/β-catenin–ferroptosis axis
Zhili Li,Claude Caron de Fromentel,Woojun Kim,Wen-Hung Wang,Jiazeng Sun,Bingyu Yan,Sagar Utturkar,Nadia Atallah Lanman,Bennett D. Elzey,Yoon Yeo,Hao Zhang,Majid Kazemian,Massimo Levrero & Ourania Andrisani
Cell Death & Disease Published:30 November 2023
DOI:https://doi.org/10.1038/s41419-023-06302-0
Abstract
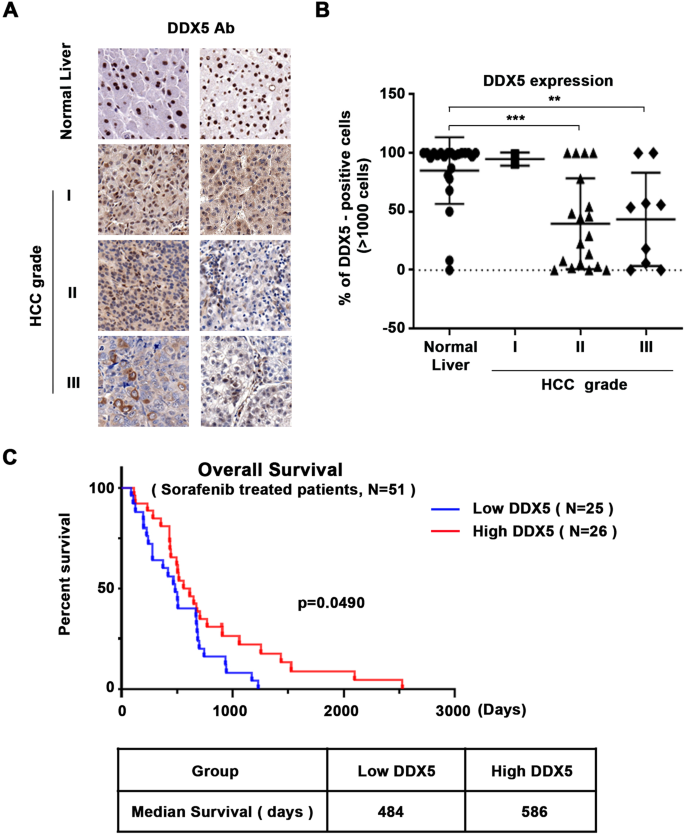
Reduced expression of the RNA helicase DDX5 associated with increased hepatocellular carcinoma (HCC) tumor grade and poor patient survival following treatment with sorafenib. While immunotherapy is the first-line treatment for HCC, sorafenib and other multi-tyrosine kinase inhibitors (mTKIs) are widely used when immunotherapy is contra-indicated or fails. Herein, we elucidate the role of DDX5 in sensitizing HCC to sorafenib, offering new therapeutic strategies. Treatment of various human HCC cell lines with sorafenib/mTKIs downregulated DDX5 in vitro and in preclinical HCC models. Conversely, DDX5 overexpression reduced the viability of sorafenib-treated cells via ferroptosis, suggesting a role for DDX5 in sorafenib sensitivity. RNAseq of wild-type vs. DDX5-knockdown cells treated with or without sorafenib identified a set of common genes repressed by DDX5 and upregulated by sorafenib. This set significantly overlaps with Wnt signaling genes, including Disheveled-1 (DVL1), an indispensable Wnt activator and prognostic indicator of poor survival for sorafenib-treated patients. DDX5-knockout (DDX5KO) HCC cells exhibited DVL1 induction, Wnt/β-catenin pathway activation, and ferroptosis upon inhibition of canonical Wnt signaling. Consistently, xenograft HCC tumors exhibited reduced growth by inhibition of Wnt/β-catenin signaling via induction of ferroptosis. Significantly, overexpression of DDX5 in HCC xenografts repressed DVL1 expression and increased ferroptosis, resulting in reduced tumor growth by sorafenib. We conclude that DDX5 downregulation by sorafenib mediates adaptive resistance by activating Wnt/β-catenin signaling, leading to ferroptosis escape. Conversely, overexpression of DDX5 in vivo enhances the anti-tumor efficacy of sorafenib by suppressing Wnt/β-catenin activation and induction of ferroptosis. Thus, DDX5 overexpression in combination with mTKIs is a promising therapeutic strategy for HCC.