2024-01-23 ワシントン州立大学(WSU)
◆妊娠中のラットの初代が共通の殺菌剤にさらされ、その子孫がジェット燃料、次いでDDTに曝露された結果、5代目の未曝露世代の動物で肥満、腎臓病、前立腺病の発生率が最大で70%増加しました。この研究は、異なる毒物に代々さらされることが、いくつかの疾患に複合的かつ増幅的な影響を与える可能性があることを示唆しています。
<関連情報>
- https://news.wsu.edu/press-release/2024/01/23/multi-generational-toxicant-exposures-show-cumulative-inherited-health-effects/
- https://academic.oup.com/eep/article/9/1/dvad006/7503815
多世代にわたる異なる有害物質曝露が、病理と肥満のエピジェネティックな世代間遺伝を引き起こす Multiple generation distinct toxicant exposures induce epigenetic transgenerational inheritance of enhanced pathology and obesity
Eric E Nilsson, Margaux McBirney, Sarah De Santos, Stephanie E King, Daniel Beck, Colin Greeley, Lawrence B Holder, Michael K Skinner
Environmental Epigenetics Published:07 December 2023
DOI:https://doi.org/10.1093/eep/dvad006
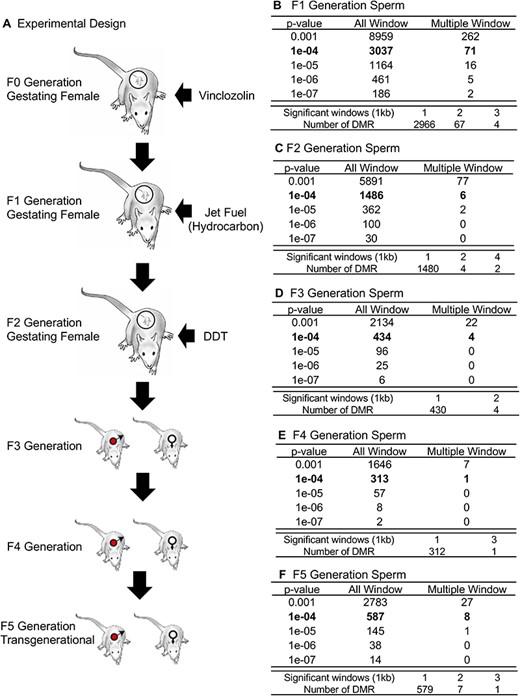
Abstract
Three successive multiple generations of rats were exposed to different toxicants and then bred to the transgenerational F5 generation to assess the impacts of multiple generation different exposures. The current study examines the actions of the agricultural fungicide vinclozolin on the F0 generation, followed by jet fuel hydrocarbon mixture exposure of the F1 generation, and then pesticide dichlorodiphenyltrichloroethane on the F2 generation gestating females. The subsequent F3 and F4 generations and F5 transgenerational generation were obtained and F1–F5 generations examined for male sperm epigenetic alterations and pathology in males and females. Significant impacts on the male sperm differential DNA methylation regions were observed. The F3–F5 generations were similar in ∼50% of the DNA methylation regions. The pathology of each generation was assessed in the testis, ovary, kidney, and prostate, as well as the presence of obesity and tumors. The pathology used a newly developed Deep Learning, artificial intelligence-based histopathology analysis. Observations demonstrated compounded disease impacts in obesity and metabolic parameters, but other pathologies plateaued with smaller increases at the F5 transgenerational generation. Observations demonstrate that multiple generational exposures, which occur in human populations, appear to increase epigenetic impacts and disease susceptibility.


