2024-03-06 バッファロー大学(UB)
<関連情報>
- https://www.buffalo.edu/news/releases/2024/03/how-fusion-proteins-hijack-gene-regulators-to-spur-childhood-cancer.html
- https://www.nature.com/articles/s41467-024-44945-5
- https://onlinelibrary.wiley.com/doi/10.1002/pro.4127
FETタンパク質のプリオン様低複雑性ドメインと哺乳類SWI/SNF複合体の選択的共縮合を異型相互作用が促進する可能性 Heterotypic interactions can drive selective co-condensation of prion-like low-complexity domains of FET proteins and mammalian SWI/SNF complex
Richoo B. Davis,Anushka Supakar,Aishwarya Kanchi Ranganath,Mahdi Muhammad Moosa & Priya R. Banerjee
Nature Communications Published:07 February 2024
DOI:https://doi.org/10.1038/s41467-024-44945-5
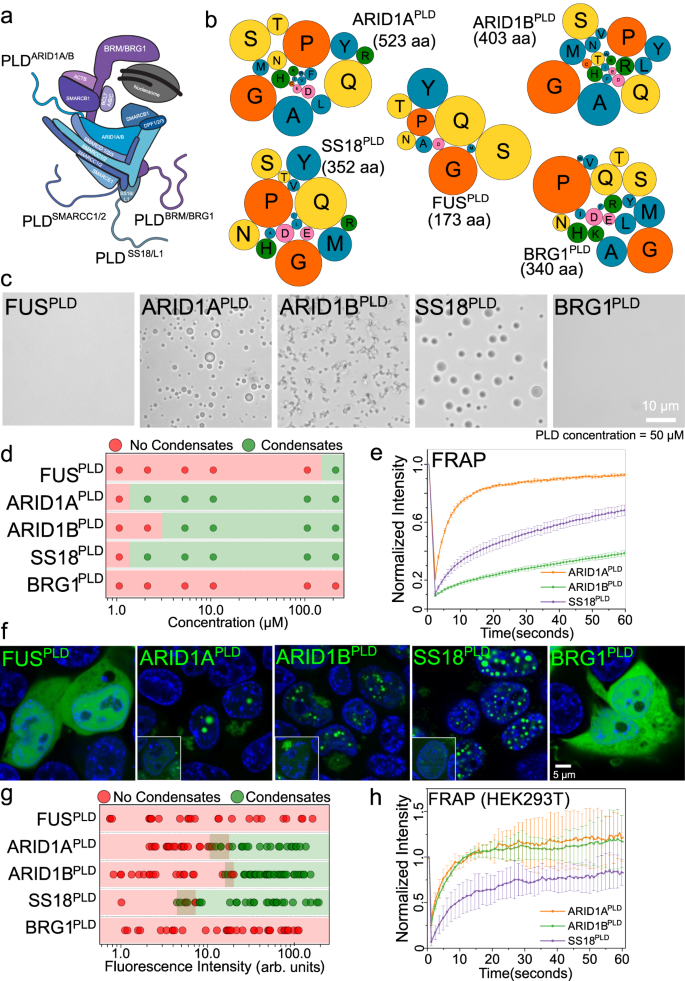
Abstract
Prion-like domains (PLDs) are low-complexity protein sequences enriched within nucleic acid-binding proteins including those involved in transcription and RNA processing. PLDs of FUS and EWSR1 play key roles in recruiting chromatin remodeler mammalian SWI/SNF (mSWI/SNF) complex to oncogenic FET fusion protein condensates. Here, we show that disordered low-complexity domains of multiple SWI/SNF subunits are prion-like with a strong propensity to undergo intracellular phase separation. These PLDs engage in sequence-specific heterotypic interactions with the PLD of FUS in the dilute phase at sub-saturation conditions, leading to the formation of PLD co-condensates. In the dense phase, homotypic and heterotypic PLD interactions are highly cooperative, resulting in the co-mixing of individual PLD phases and forming spatially homogeneous condensates. Heterotypic PLD-mediated positive cooperativity in protein-protein interaction networks is likely to play key roles in the co-phase separation of mSWI/SNF complex with transcription factors containing homologous low-complexity domains.
FUSオンコフュージョンタンパク質は、プリオン様ドメイン間のヘテロ型相互作用を介してmSWI/SNFクロマチンリモデラーをリクルートする FUS oncofusion protein condensates recruit mSWI/SNF chromatin remodeler via heterotypic interactions between prion-like domains
Richoo B. Davis, Taranpreet Kaur, Mahdi Muhammad Moosa, Priya R. Banerjee
Protein Science Published: 21 May 2021
DOI:https://doi.org/10.1002/pro.4127

Abstract
Fusion transcription factors generated by genomic translocations are common drivers of several types of cancers including sarcomas and leukemias. Oncofusions of the FET (FUS, EWSR1, and TAF15) family proteins result from the fusion of the prion-like domain (PLD) of FET proteins to the DNA-binding domain (DBD) of certain transcription regulators and are implicated in aberrant transcriptional programs through interactions with chromatin remodelers. Here, we show that FUS-DDIT3, a FET oncofusion protein, undergoes PLD-mediated phase separation into liquid-like condensates. Nuclear FUS-DDIT3 condensates can recruit essential components of the global transcriptional machinery such as the chromatin remodeler SWI/SNF. The recruitment of mammalian SWI/SNF (mSWI/SNF) is driven by heterotypic PLD-PLD interactions between FUS-DDIT3 and core subunits of SWI/SNF, such as the catalytic component BRG1. Further experiments with single-molecule correlative force-fluorescence microscopy support a model wherein the fusion protein forms condensates on DNA surface and enrich BRG1 to activate transcription by ectopic chromatin remodeling. Similar PLD-driven co-condensation of mSWI/SNF with transcription factors can be employed by other oncogenic fusion proteins with a generic PLD-DBD domain architecture for global transcriptional reprogramming.