2024-03-28 ワシントン大学セントルイス校
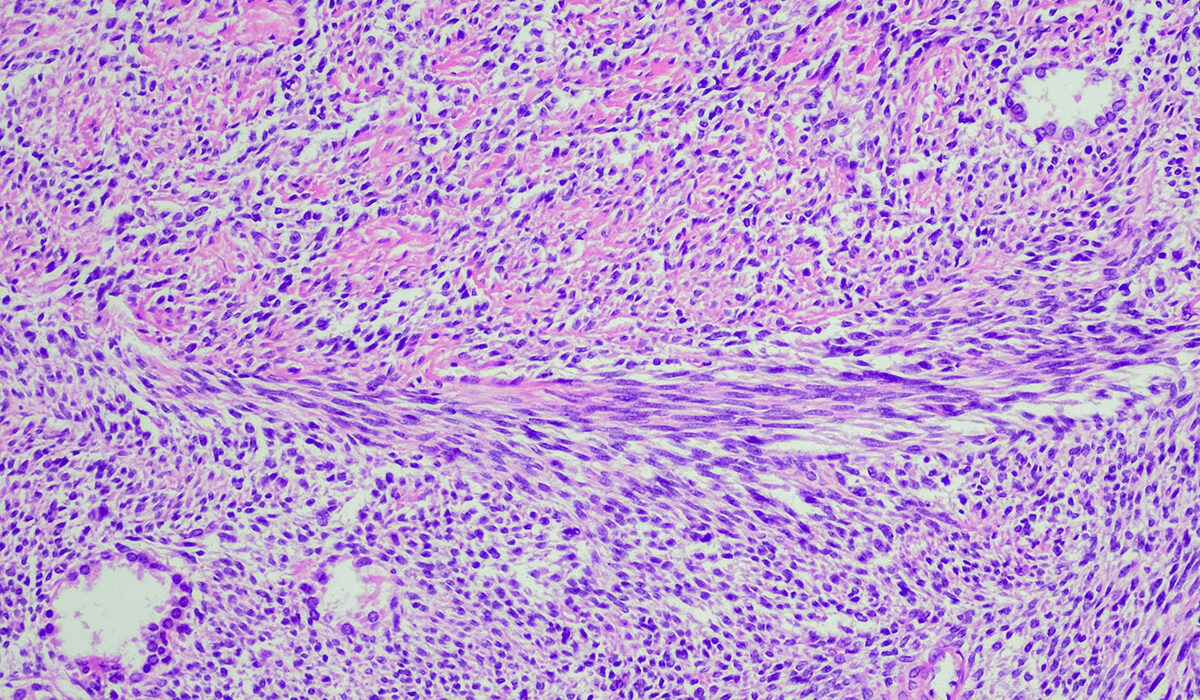
According to a study led by Washington University School of Medicine in St. Louis, a new T cell immunotherapy — in which the patients’ own T cells are genetically modified to attack and kill cancer cells — is effective in treating some patients with two types of sarcoma, rare cancers of the body’s soft tissues. Shown is a cross section of a synovial sarcoma tumor. (Image: Getty Images)
<関連情報>
- https://source.wustl.edu/2024/03/some-sarcoma-patients-improve-with-t-cell-immunotherapy/
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(24)00319-2/abstract
進行滑膜肉腫および粘液様円形細胞脂肪肉腫に対するAfamitresgene autoleucel(SPEARHEAD-1):国際非盲検第2相試験 Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): an international, open-label, phase 2 trial
Sandra P D’Angelo, MD ;Prof Dejka M Araujo, MD;Albiruni R Abdul Razak, MBBCh;Prof Mark Agulnik, MD;Steven Attia, DO;Prof Jean-Yves Blay, MD;et a
The Lancet Published:March 27, 2024
DOI:https://doi.org/10.1016/S0140-6736(24)00319-2
Summary
Background
Afamitresgene autoleucel (afami-cel) showed acceptable safety and promising efficacy in a phase 1 trial (NCT03132922). The aim of this study was to further evaluate the efficacy of afami-cel for the treatment of patients with HLA-A*02 and MAGE-A4-expressing advanced synovial sarcoma or myxoid round cell liposarcoma.
Methods
SPEARHEAD-1 was an open-label, non-randomised, phase 2 trial done across 23 sites in Canada, the USA, and Europe. The trial included three cohorts, of which the main investigational cohort (cohort 1) is reported here. Cohort 1 included patients with HLA-A*02, aged 16–75 years, with metastatic or unresectable synovial sarcoma or myxoid round cell liposarcoma (confirmed by cytogenetics) expressing MAGE-A4, and who had received at least one previous line of anthracycline-containing or ifosfamide-containing chemotherapy. Patients received a single intravenous dose of afami-cel (transduced dose range 1·0 × 109–10·0 × 109 T cells) after lymphodepletion. The primary endpoint was overall response rate in cohort 1, assessed by a masked independent review committee using Response Evaluation Criteria in Solid Tumours (version 1.1) in the modified intention-to-treat population (all patients who received afami-cel). Adverse events, including those of special interest (cytokine release syndrome, prolonged cytopenia, and neurotoxicity), were monitored and are reported for the modified intention-to-treat population. This trial is registered at ClinicalTrials.gov, NCT04044768; recruitment is closed and follow-up is ongoing for cohorts 1 and 2, and recruitment is open for cohort 3.
Findings
Between Dec 17, 2019, and July 27, 2021, 52 patients with cytogenetically confirmed synovial sarcoma (n=44) and myxoid round cell liposarcoma (n=8) were enrolled and received afami-cel in cohort 1. Patients were heavily pre-treated (median three [IQR two to four] previous lines of systemic therapy). Median follow-up time was 32·6 months (IQR 29·4–36·1). Overall response rate was 37% (19 of 52; 95% CI 24–51) overall, 39% (17 of 44; 24–55) for patients with synovial sarcoma, and 25% (two of eight; 3–65) for patients with myxoid round cell liposarcoma. Cytokine release syndrome occurred in 37 (71%) of 52 of patients (one grade 3 event). Cytopenias were the most common grade 3 or worse adverse events (lymphopenia in 50 [96%], neutropenia 44 [85%], leukopenia 42 [81%] of 52 patients). No treatment-related deaths occurred.
Interpretation
Afami-cel treatment resulted in durable responses in heavily pre-treated patients with HLA-A*02 and MAGE-A4-expressing synovial sarcoma. This study shows that T-cell receptor therapy can be used to effectively target solid tumours and provides rationale to expand this approach to other solid malignancies.
Funding
Adaptimmune.