2024-06-07 ニューサウスウェールズ大学(UNSW)
<関連情報>
- https://www.unsw.edu.au/newsroom/news/2024/06/precision-medicine-for-childhood-cancer-improves-survival-trial
- https://www.nature.com/articles/s41591-024-03044-0
高リスクの小児がんにおける精密誘導治療 Precision-guided treatment in high-risk pediatric cancers
Loretta M. S. Lau,Dong-Anh Khuong-Quang,Chelsea Mayoh,Marie Wong,Paulette Barahona,Pamela Ajuyah,Akanksha Senapati,Sumanth Nagabushan,Alexandra Sherstyuk,Ann-Kristin Altekoester,Noemi A. Fuentes-Bolanos,Veronica Yeung,Ashleigh Sullivan,Natacha Omer,Yonatan Diamond,Sophie Jessop,Lauren Battaglia,Nataliya Zhukova,Louise Cui,Angela Lin,Andrew J. Gifford,Emmy D. G. Fleuren,Luciano Dalla-Pozza,Andrew S. Moore,… David S. Ziegler
Nature Medicine Published:06 June 2024
DOI:https://doi.org/10.1038/s41591-024-03044-0
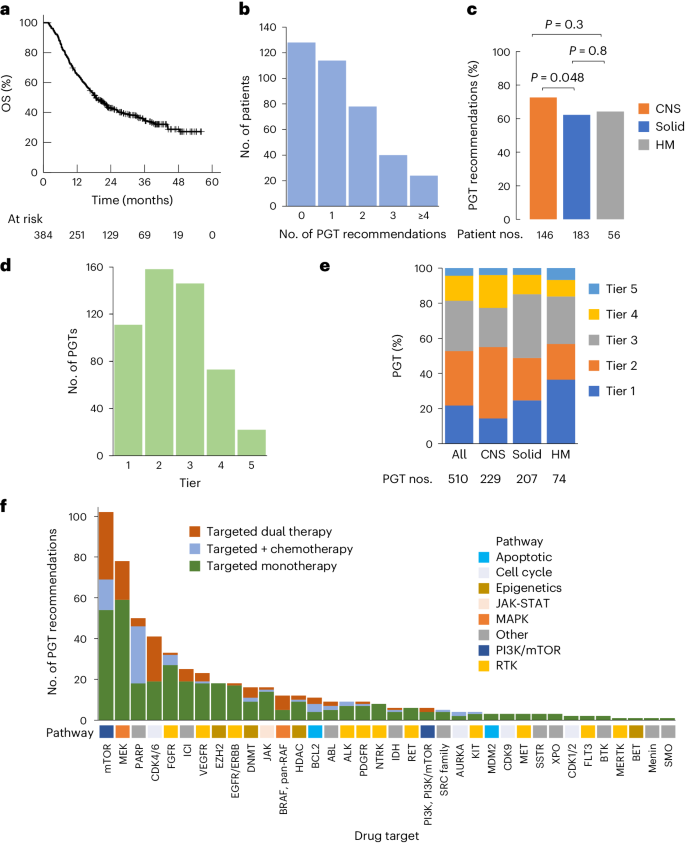
Abstract
Recent research showed that precision medicine can identify new treatment strategies for patients with childhood cancers. However, it is unclear which patients will benefit most from precision-guided treatment (PGT). Here we report consecutive data from 384 patients with high-risk pediatric cancer (with an expected cure rate of less than 30%) who had at least 18 months of follow-up on the ZERO Childhood Cancer Precision Medicine Program PRecISion Medicine for Children with Cancer (PRISM) trial. A total of 256 (67%) patients received PGT recommendations and 110 (29%) received a recommended treatment. PGT resulted in a 36% objective response rate and improved 2-year progression-free survival compared with standard of care (26% versus 12%; P = 0.049) or targeted agents not guided by molecular findings (26% versus 5.2%; P = 0.003). PGT based on tier 1 evidence, PGT targeting fusions or commenced before disease progression had the greatest clinical benefit. Our data show that PGT informed by comprehensive molecular profiling significantly improves outcomes for children with high-risk cancers. ClinicalTrials.gov registration: NCT03336931

