2024-08-20 カナダ・ブリティッシュコロンビア大学(UBC)
<関連情報>
- https://news.ubc.ca/2024/08/how-early-life-antibiotics-turn-immunity-into-allergy/
- https://www.jacionline.org/article/S0091-6749(24)00781-4/pdf
腸内細菌異常症は、ILC2-B1細胞-宿主IgE軸を形成することで、長期的なアレルギー感受性を引き起こす Microbial intestinal dysbiosis drives long-term allergic susceptibility by sculpting an ILC2-B1 cell-innate IgE axis
Ahmed Kabil, Natalia Nayyar, Julyanne Brassard, Yicong Li, Sameeksha Chopra, Michael R Hughes, Kelly M McNagny
Journal of Allergy and Clinical Immunology Accepted Date: 19 July 2024
DOI:https://doi.org/10.1016/j.jaci.2024.07.023
Abstract
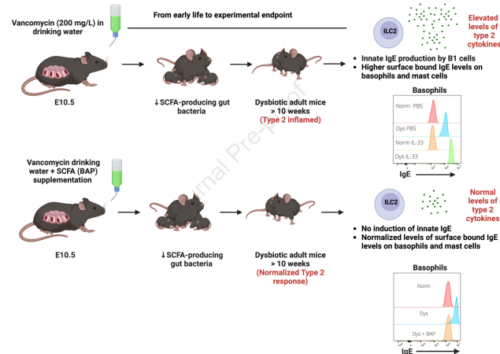
Background: The abundance and diversity of intestinal commensal bacteria influence systemic immunity with impact on disease susceptibility and severity. For example, loss of short chain fatty acid (SCFA)-fermenting bacteria in early life (humans and mice) is associated with enhanced type 2 immune responses in peripheral tissues including the lung.
Objective: Our goal was to reveal the microbiome-dependent cellular and molecular mechanisms driving enhanced susceptibility to type 2 allergic lung disease.
Methods: We used low-dose vancomycin to selectively deplete SCFA-fermenting bacteria in wild type mice (Vanc-dys mice). We then examined the frequency and activation status of innate and adaptive immune cell lineages with and without SCFA supplementation. Finally, we used ILC2-deficient and signal transducer and activator of transcription 6 (STAT6)-deficient transgenic mouse strains to delineate the cellular and cytokine pathways leading to enhanced allergic disease susceptibility.
Results: Vanc-dys mice exhibit a 2-fold increase in lung ILC2 primed to produce elevated levels of interleukin (IL)-2, -5 and -13. In addition, upon IL-33 treatment, Vanc-dys lung ILC2 display a novel ability to produce high levels of IL-4. These expanded and primed ILC2 drive B1 cell expansion and IL-4-dependent production of IgE that, in turn, leads to exacerbated allergic inflammation. Importantly, these enhanced lung inflammatory phenotypes in Vanc-dys mice were reversed by administration of dietary SCFA (specifically butyrate).
Conclusion: SCFA regulate an ILC2-B1cell-IgE axis. Early-life administration of vancomycin, an antibiotic known to deplete SCFA-fermenting gut bacteria, primes and amplifies this axis and leads to a lifelong enhanced susceptibility to type 2 allergic lung disease.
Keywords: B1 cells; ILC2; allergic lung inflammation; gut microbiome; innate natural IgE; short-chain fatty acids.