2024-10-11 オランダ・デルフト工科大学(TUDelft)
<関連情報>
- https://www.tudelft.nl/en/2024/tnw/delft-scientists-discover-how-innate-immunity-envelops-bacteria
- https://www.nature.com/articles/s41594-024-01400-9
ヒトGBP1による抗菌膜コート形成の構造的基盤 Structural basis of antimicrobial membrane coat assembly by human GBP1
Tanja Kuhm,Clémence Taisne,Cecilia de Agrela Pinto,Luca Gross,Evdokia A. Giannopoulou,Stefan T. Huber,Els Pardon,Jan Steyaert,Sander J. Tans & Arjen J. Jakobi
Nature Structural & Molecular Biology Published:11 October 2024
DOI:https://doi.org/10.1038/s41594-024-01400-9
Abstract
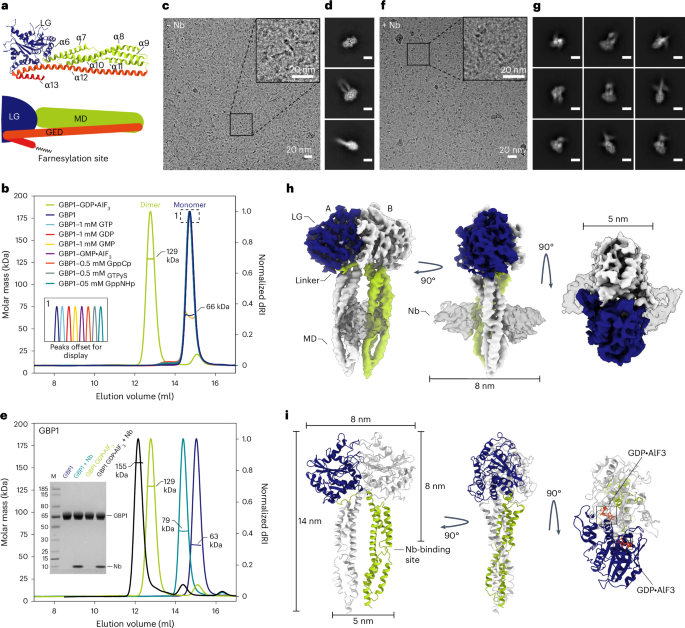
Guanylate-binding proteins (GBPs) are interferon-inducible guanosine triphosphate hydrolases (GTPases) mediating host defense against intracellular pathogens. Their antimicrobial activity hinges on their ability to self-associate and coat pathogen-associated compartments or cytosolic bacteria. Coat formation depends on GTPase activity but how nucleotide binding and hydrolysis prime coat formation remains unclear. Here, we report the cryo-electron microscopy structure of the full-length human GBP1 dimer in its guanine nucleotide-bound state and describe the molecular ultrastructure of the GBP1 coat on liposomes and bacterial lipopolysaccharide membranes. Conformational changes of the middle and GTPase effector domains expose the isoprenylated C terminus for membrane association. The α-helical middle domains form a parallel, crossover arrangement essential for coat formation and position the extended effector domain for intercalation into the lipopolysaccharide layer of gram-negative membranes. Nucleotide binding and hydrolysis create oligomeric scaffolds with contractile abilities that promote membrane extrusion and fragmentation. Our data offer a structural and mechanistic framework for understanding GBP1 effector functions in intracellular immunity.