2024-11-20 カナダ・ブリティッシュコロンビア大学(UBC)
<関連情報>
- https://news.ubc.ca/2024/11/cancer-like-mutations-in-healthy-cells-point-to-origins-of-breast-cancer/
- https://www.nature.com/articles/s41588-024-01988-0
BRCA1またはBRCA2変異保因者と非保因者の乳房内腔上皮細胞は、乳癌共通のコピー数変化を保持している Luminal breast epithelial cells of BRCA1 or BRCA2 mutation carriers and noncarriers harbor common breast cancer copy number alterations
Marc J. Williams,Michael U. J. Oliphant,Vinci Au,Cathy Liu,Caroline Baril,Ciara O’Flanagan,Daniel Lai,Sean Beatty,Michael Van Vliet,Jacky CH Yiu,Lauren O’Connor,Walter L. Goh,Alicia Pollaci,Adam C. Weiner,Diljot Grewal,Andrew McPherson,Klarisa Norton,McKenna Moore,Vikas Prabhakar,Shailesh Agarwal,Judy E. Garber,Deborah A. Dillon,Sohrab P. Shah,Joan S. Brugge & Samuel Aparicio
Nature Genetics Published:20 November 2024
DOI:https://doi.org/10.1038/s41588-024-01988-0
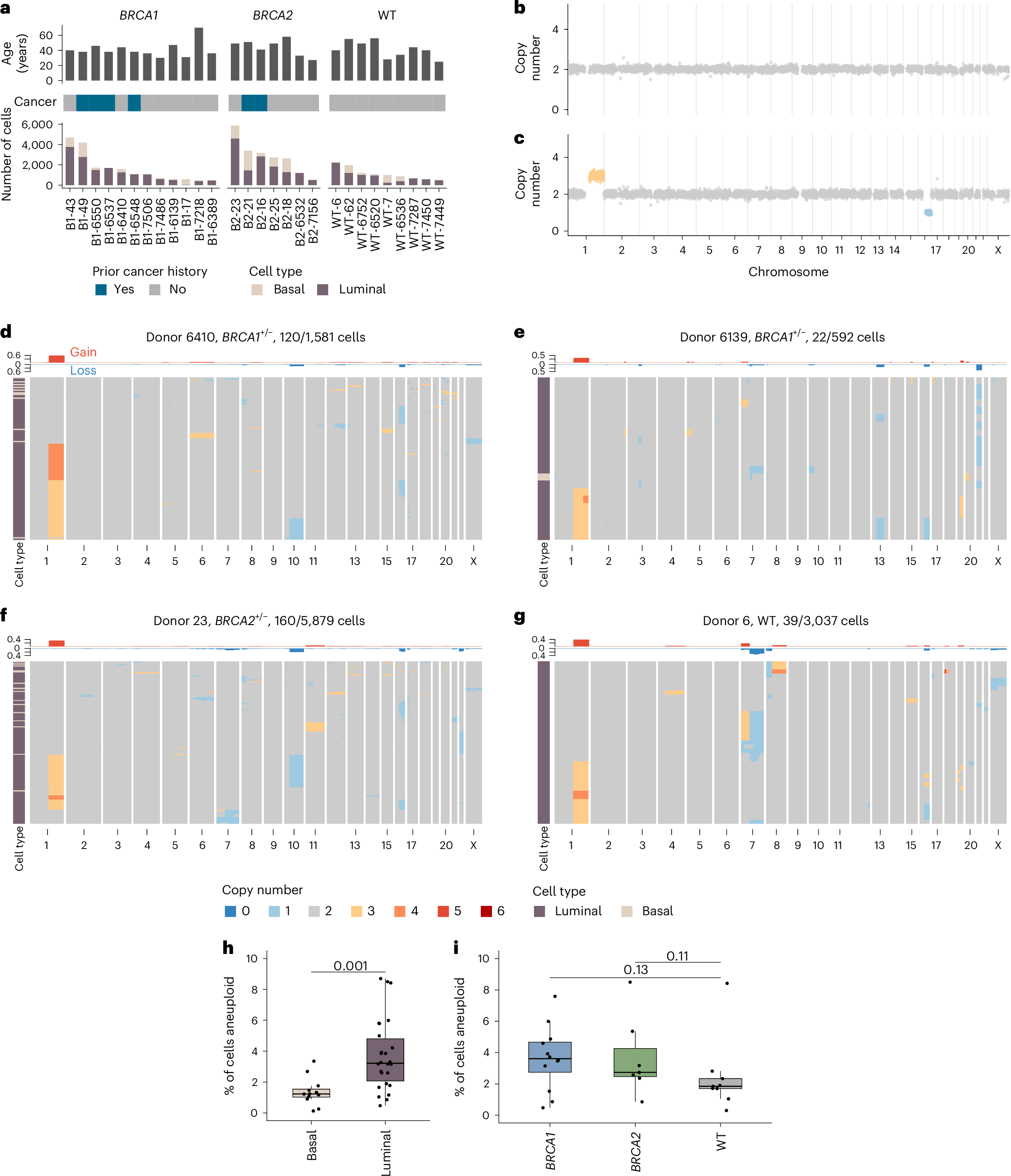
Abstract
The prevalence and nature of somatic copy number alterations (CNAs) in breast epithelium and their role in tumor initiation and evolution remain poorly understood. Using single-cell DNA sequencing (49,238 cells) of epithelium from BRCA1 and BRCA2 carriers or wild-type individuals, we identified recurrent CNAs (for example, 1q-gain and 7q, 10q, 16q and 22q-loss) that are present in a rare population of cells across almost all samples (n = 28). In BRCA1/BRCA2 carriers, these occur before loss of heterozygosity (LOH) of wild-type alleles. These CNAs, common in malignant tumors, are enriched in luminal cells but absent in basal myoepithelial cells. Allele-specific analysis of prevalent CNAs reveals that they arose by independent mutational events, consistent with convergent evolution. BRCA1/BRCA2 carriers contained a small percentage of cells with extreme aneuploidy, featuring loss of TP53, BRCA1/BRCA2 LOH and multiple breast cancer-associated CNAs. Our findings suggest that CNAs arising in normal luminal breast epithelium are precursors to clonally expanded tumor genomes.