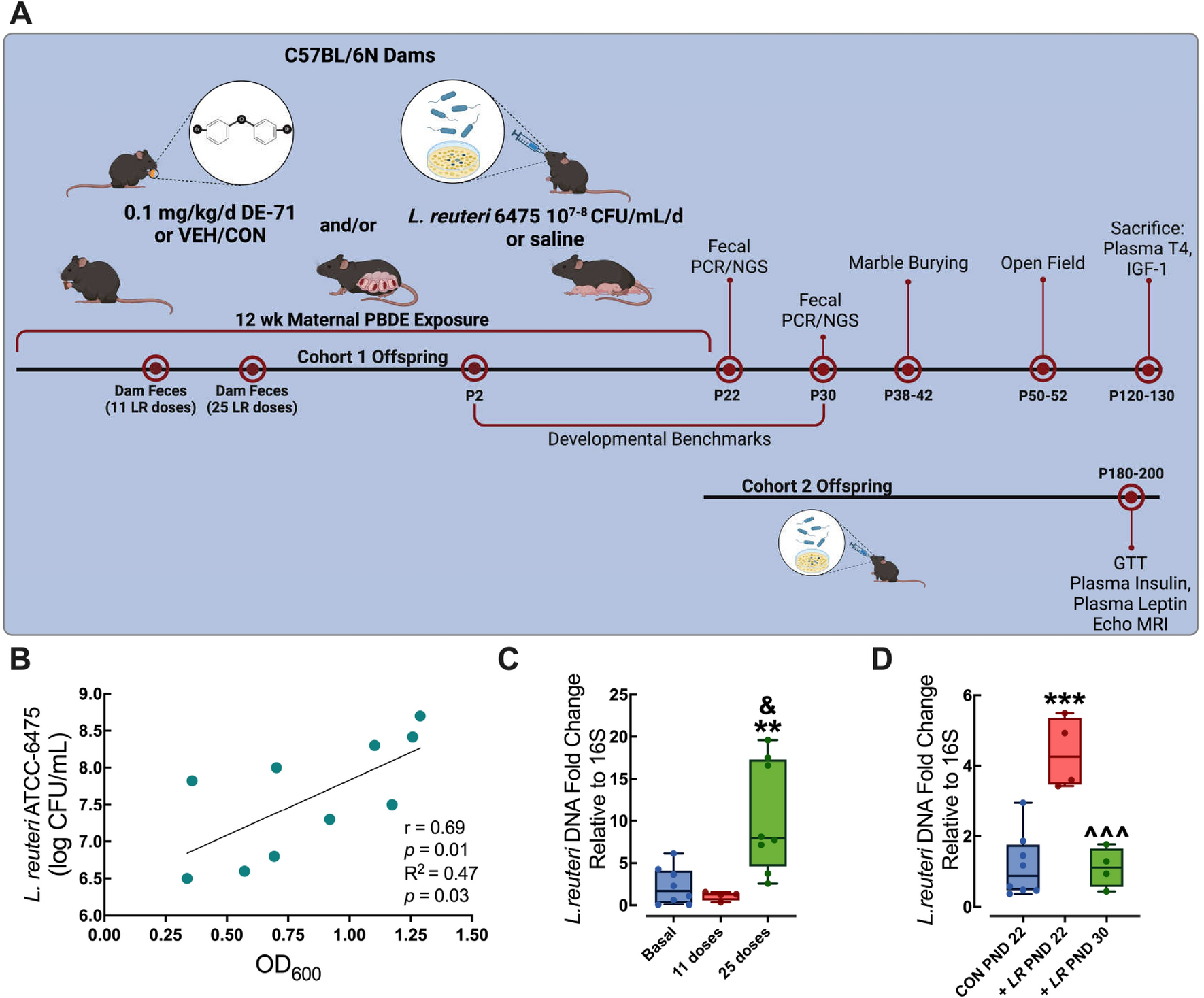
2024-11-20 コロンビア大学
<関連情報>
- https://www.cuimc.columbia.edu/news/immune-memory-covid-19-vaccination-stored-throughout-body
- https://www.cell.com/immunity/abstract/S1074-7613(24)00483-7
組織におけるCOVID-19ワクチンに対する免疫記憶の維持と機能制御 Maintenance and functional regulation of immune memory to COVID-19 vaccines in tissues
Julia Davis-Porada, ∙ Alex B. George∙ Nora Lam∙ … ∙ John R. Teijaro∙ Peter A. Sims∙ Donna L. Farber
Immunity Published:November 6, 2024
DOI:https://doi.org/10.1016/j.immuni.2024.10.003
Graphical abstract

Highlights
•Vaccine-induced memory T and B cells persist in lymphoid organs, lungs, and blood
•Memory T cells in tissues are more durably maintained compared with blood
•Tissue-resident spike-reactive memory T cells are optimally generated by infection
•Spike-reactive T cells exhibit an enhanced regulatory profile in certain tissues
Summary
Memory T and B cells in tissues are essential for protective immunity. Here, we performed a comprehensive analysis of the tissue distribution, phenotype, durability, and transcriptional profile of COVID-19 mRNA vaccine-induced immune memory across blood, lymphoid organs, and lungs obtained from 63 vaccinated organ donors aged 23–86, some of whom experienced SARS-CoV-2 infection. Spike (S)-reactive memory T cells were detected in lymphoid organs and lungs and variably expressed tissue-resident markers based on infection history, and S-reactive B cells comprised class-switched memory cells resident in lymphoid organs. Compared with blood, S-reactive tissue memory T cells persisted for longer times post-vaccination and were more prevalent with age. S-reactive T cells displayed site-specific subset compositions and functions: regulatory cell profiles were enriched in tissues, while effector and cytolytic profiles were more abundant in circulation. Our findings reveal functional compartmentalization of vaccine-induced T cell memory where surveilling effectors and in situ regulatory responses confer protection with minimal tissue damage.