2025-01-08 カリフォルニア大学リバーサイド校
<関連情報>
- https://news.ucr.edu/articles/2025/01/08/study-links-gene-regulating-brain-circuit-formation-autism-and-seizures
- https://www.nature.com/articles/s41380-024-02839-4
抑制性ニューロンにおけるニューロピリン2発現の調節異常は、海馬回路の発達を障害し、自閉症関連行動および発作のリスクを高める Dysregulation of neuropilin-2 expression in inhibitory neurons impairs hippocampal circuit development and enhances risk for autism-related behaviors and seizures
Deepak Subramanian,Carol Eisenberg,Andrew Huang,Jiyeon Baek,Haniya Naveed,Samiksha Komatireddy,Michael W. Shiflett,Tracy S. Tran & Vijayalakshmi Santhakumar
Molecular Psychiatry Published:22 November 2024
DOI:https://doi.org/10.1038/s41380-024-02839-4
Abstract
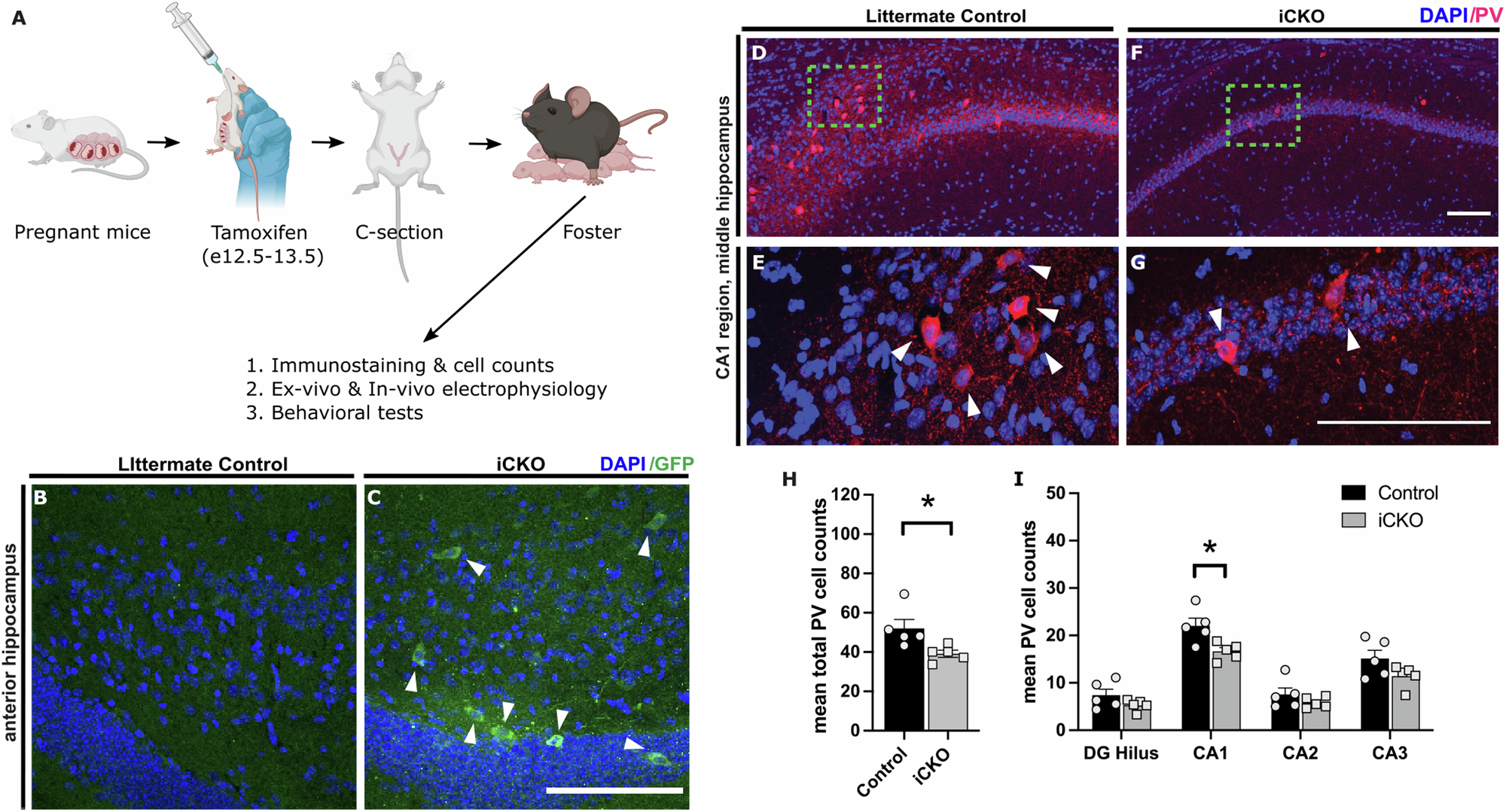
Dysregulation of development, migration, and function of interneurons, collectively termed interneuronopathies, have been proposed as a shared mechanism for autism spectrum disorders (ASDs) and childhood epilepsy. Neuropilin-2 (Nrp2), a candidate ASD gene, is a critical regulator of interneuron migration from the median ganglionic eminence (MGE) to the pallium, including the hippocampus. While clinical studies have identified Nrp2 polymorphisms in patients with ASD, whether selective dysregulation of Nrp2-dependent interneuron migration contributes to pathogenesis of ASD and enhances the risk for seizures has not been evaluated. We tested the hypothesis that the lack of Nrp2 in MGE-derived interneuron precursors disrupts the excitation/inhibition balance in hippocampal circuits, thus predisposing the network to seizures and behavioral patterns associated with ASD. Embryonic deletion of Nrp2 during the developmental period for migration of MGE derived interneuron precursors (iCKO) significantly reduced parvalbumin, neuropeptide Y, and somatostatin positive neurons in the hippocampal CA1. Consequently, when compared to controls, the frequency of inhibitory synaptic currents in CA1 pyramidal cells was reduced while frequency of excitatory synaptic currents was increased in iCKO mice. Although passive and active membrane properties of CA1 pyramidal cells were unchanged, iCKO mice showed enhanced susceptibility to chemically evoked seizures. Moreover, iCKO mice exhibited selective behavioral deficits in both preference for social novelty and goal-directed learning, which are consistent with ASD-like phenotype. Together, our findings show that disruption of developmental Nrp2 regulation of interneuron circuit establishment, produces ASD-like behaviors and enhanced risk for epilepsy. These results support the developmental interneuronopathy hypothesis of ASD epilepsy comorbidity.

