2025-03-24 ワシントン大学セントルイス校(WashU)
<関連情報>
- https://source.washu.edu/2025/03/the-right-moves-to-rein-in-fibrosis/
- https://www.nature.com/articles/s41563-025-02162-5
- https://www.cell.com/iscience/fulltext/S2589-0042(24)01176-3
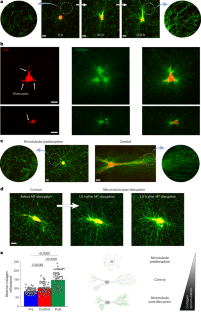
張力異方性が細胞-細胞外マトリックスの力学的フィードバックを自己強化することで線維芽細胞の表現型転移を駆動する Tension anisotropy drives fibroblast phenotypic transition by self-reinforcing cell–extracellular matrix mechanical feedback
Farid Alisafaei,Delaram Shakiba,Yuan Hong,Ghiska Ramahdita,Yuxuan Huang,Leanne E. Iannucci,Matthew D. Davidson,Mohammad Jafari,Jin Qian,Chengqing Qu,David Ju,Dashiell R. Flory,Yin-Yuan Huang,Prashant Gupta,Shumeng Jiang,Aliza Mujahid,Srikanth Singamaneni,Kenneth M. Pryse,Pen-hsiu Grace Chao,Jason A. Burdick,Spencer P. Lake,Elliot L. Elson,Nathaniel Huebsch,Vivek B. Shenoy & Guy M. Genin
Nature Materials Published:24 March 2025
DOI:https://doi.org/10.1038/s41563-025-02162-5
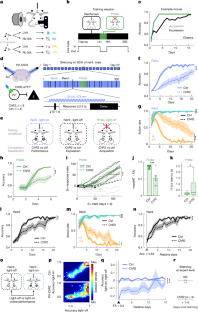
Abstract
Mechanical factors such as stress in the extracellular environment affect the phenotypic commitment of cells. Stress fields experienced by cells in tissues are multiaxial, but how cells integrate such information is largely unknown. Here we report that the anisotropy of stress fields is a critical factor triggering a phenotypic transition in fibroblast cells, outweighing the role of stress amplitude, a factor previously described to modulate such a transition. Combining experimental and computational approaches, we identified a self-reinforcing mechanism in which cellular protrusions interact with collagen fibres to establish tension anisotropy. This anisotropy, in turn, stabilizes the protrusions and enhances their contractile forces. Disruption of this self-reinforcing process, either by reducing tension anisotropy or by inhibiting contractile protrusions, prevents the phenotypic conversion of fibroblasts to contractile myofibroblasts. Overall, our findings support stress anisotropy as a factor modulating cellular responses, expanding our understanding of the role of mechanical forces in biological processes.
肥大型心筋症のiPSCマイクロ組織モデルにおいて、基質力学が初期の構造的・機能的病態を明らかにする Substrate mechanics unveil early structural and functional pathology in iPSC micro-tissue models of hypertrophic cardiomyopathy
Jingxuan Guo ∙ Huanzhu Jiang ∙ David Schuftan∙ … ∙ Druv Bhagavan ∙ Jonathan Silva ∙ Nathaniel Huebsch
iScience Published:May 9, 2024
DOI:https://doi.org/10.1016/j.isci.2024.109954
Graphical abstract

Highlights
- Substrate mechanics and MYBPC3+/- mutation trigger early hallmarks of HCM using iPSC
- Differential troponin complex localization was observed in MYBPC3+/- tissues
- MYBPC3+/- tissues exhibit impaired contractile energetics and slower kinetics
- Excessive channel activity underlies abnormal Ca2+ handling in MYBPC3+/- tissues
Summary
Hypertension is a major cause of morbidity and mortality in patients with hypertrophic cardiomyopathy (HCM), suggesting a potential role for mechanics in HCM pathogenesis. Here, we developed an in vitro physiological model to investigate how mechanics acts together with HCM-linked myosin binding protein C (MYBPC3) mutations to trigger disease. Micro-heart muscles (μHM) were engineered from induced pluripotent stem cell (iPSC)-derived cardiomyocytes bearing MYBPC3+/- mutations and challenged to contract against substrates of different elasticity. μHMs that worked against substrates with stiffness at or exceeding the stiffness of healthy adult heart muscle exhibited several hallmarks of HCM, including cellular hypertrophy, impaired contractile energetics, and maladaptive calcium handling. Remarkably, we discovered changes in troponin C and T localization in MYBPC3+/- μHM that were entirely absent in 2D culture. Pharmacologic studies suggested that excessive Ca2+ intake through membrane-embedded channels underlie the observed electrophysiological abnormalities. These results illustrate the power of physiologically relevant engineered tissue models to study inherited disease with iPSC technology.