2025-04-03 京都大学iPS細胞研究所
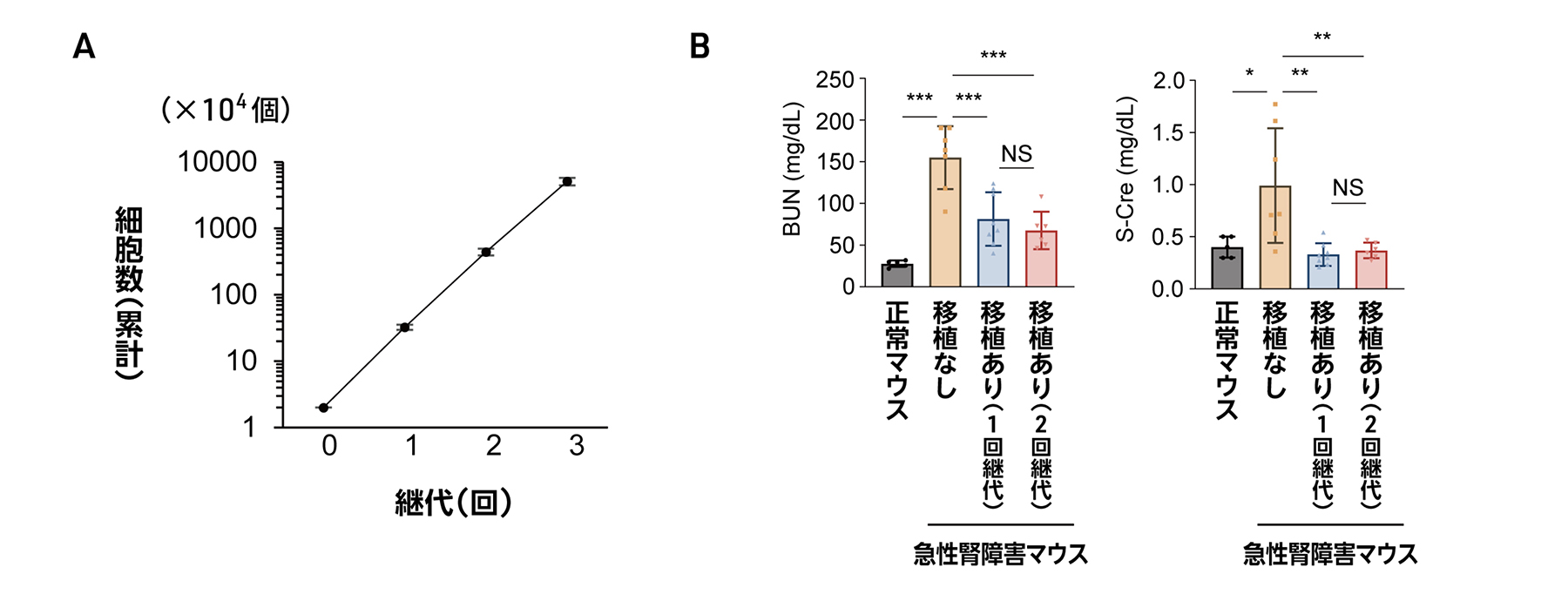
図1 CFY培養液を用いた腎前駆細胞の継代
<関連情報>
- https://www.cira.kyoto-u.ac.jp/j/pressrelease/news/250403-030000.html
- https://www.science.org/doi/10.1126/scitranslmed.adt5553
ヒトiPSC由来ネフロン前駆細胞がマウスモデルで急性腎障害と慢性腎臓病を治療する Human iPSC–derived nephron progenitor cells treat acute kidney injury and chronic kidney disease in mouse models
Toshikazu Araoka, Kosuke Toyohara, Makoto Ryosaka, Chihiro Inui, […], and Kenji Osafune
Science Translational Medicine Published:2 Apr 2025
DOI:https://doi.org/10.1126/scitranslmed.adt5553
Editor’s summary
Cell-based therapies for kidney disease have not been clinically validated, and methods require large numbers of cells for treatment. Araoka et al. created a cell culture medium to expand human induced pluripotent stem cell–derived nephron progenitor cells (hiPSC-NPCs) for treating acute kidney injury (AKI) and chronic kidney disease (CKD) in mouse models. The culture medium allowed 100-fold expansion of cells. Implantation of the hiPSC-NPCs attenuated kidney functional decline in both AKI and CKD models. Conditioned medium from giant cell aggregates from the expanded hiPSC-NPCs was also able to treat kidney pathology in these models. Secreted vascular endothelial growth factor A (VEGF-A) was partially responsible for the beneficial effects, given that genetic silencing of VEGF-A in the cells blunted therapeutic effects. Together, these results support further study of cell-based therapies for AKI and CKD. —Brandon Berry
Abstract
The number of patients requiring dialysis therapy continues to increase worldwide because of the lack of effective treatments for chronic kidney disease (CKD). Furthermore, no curative treatments for acute kidney injury (AKI) have been established. The therapeutic effects of human induced pluripotent stem cell–derived nephron progenitor cells (hiPSC-NPCs) on AKI have been reported in mice but not clinically confirmed. There are also no reports examining the therapeutic potential of hiPSC-NPCs on CKD. Although large numbers of uniform hiPSC-NPCs are required for cell therapies for AKI and CKD, effective expansion cultures remain to be developed. Here, we established a culture medium for cells that enabled more than 100-fold proliferation of hiPSC-NPCs from multiple hiPSC lines in two passages. We demonstrated that hiPSC-NPCs expanded by our medium named CFY or by their conditioned medium alone attenuated kidney injury and improved survival in cisplatin-induced AKI mice. We also observed that hiPSC-NPCs prevented kidney functional decline, interstitial fibrosis, and senescence in aristolochic acid–induced CKD mice. In addition, we found c-MET to be a specific cell surface marker for hiPSC-NPCs and confirmed that purified c-MET+ hiPSC-NPCs had therapeutic effects on AKI and CKD mice. Furthermore, we found that hiPSC-NPCs exerted their therapeutic effects in AKI and CKD mice by secreting vascular endothelial growth factor A. Expanded hiPSC-NPCs may be useful cell therapies for AKI and CKD and may open avenues for treating kidney diseases.


