2025-04-07 タフツ大学
<関連情報>
- https://now.tufts.edu/2025/04/07/alternative-approach-lyme-disease-vaccine-development-shows-promise-pre-clinical-models
- https://www.nature.com/articles/s41467-025-58182-x
CspZを標的としたライム病ワクチンの構造に基づく設計に関するメカニズム的洞察 Mechanistic insights into the structure-based design of a CspZ-targeting Lyme disease vaccine
Kalvis Brangulis,Jill Malfetano,Ashley L. Marcinkiewicz,Alan Wang,Yi-Lin Chen,Jungsoon Lee,Zhuyun Liu,Xiuli Yang,Ulrich Strych,Dagnija Tupina,Inara Akopjana,Maria-Elena Bottazzi,Utpal Pal,Ching-Lin Hsieh,Wen-Hsiang Chen & Yi-Pin Lin
Nature Communications Published:07 April 2025
DOI:https://doi.org/10.1038/s41467-025-58182-x
Abstract
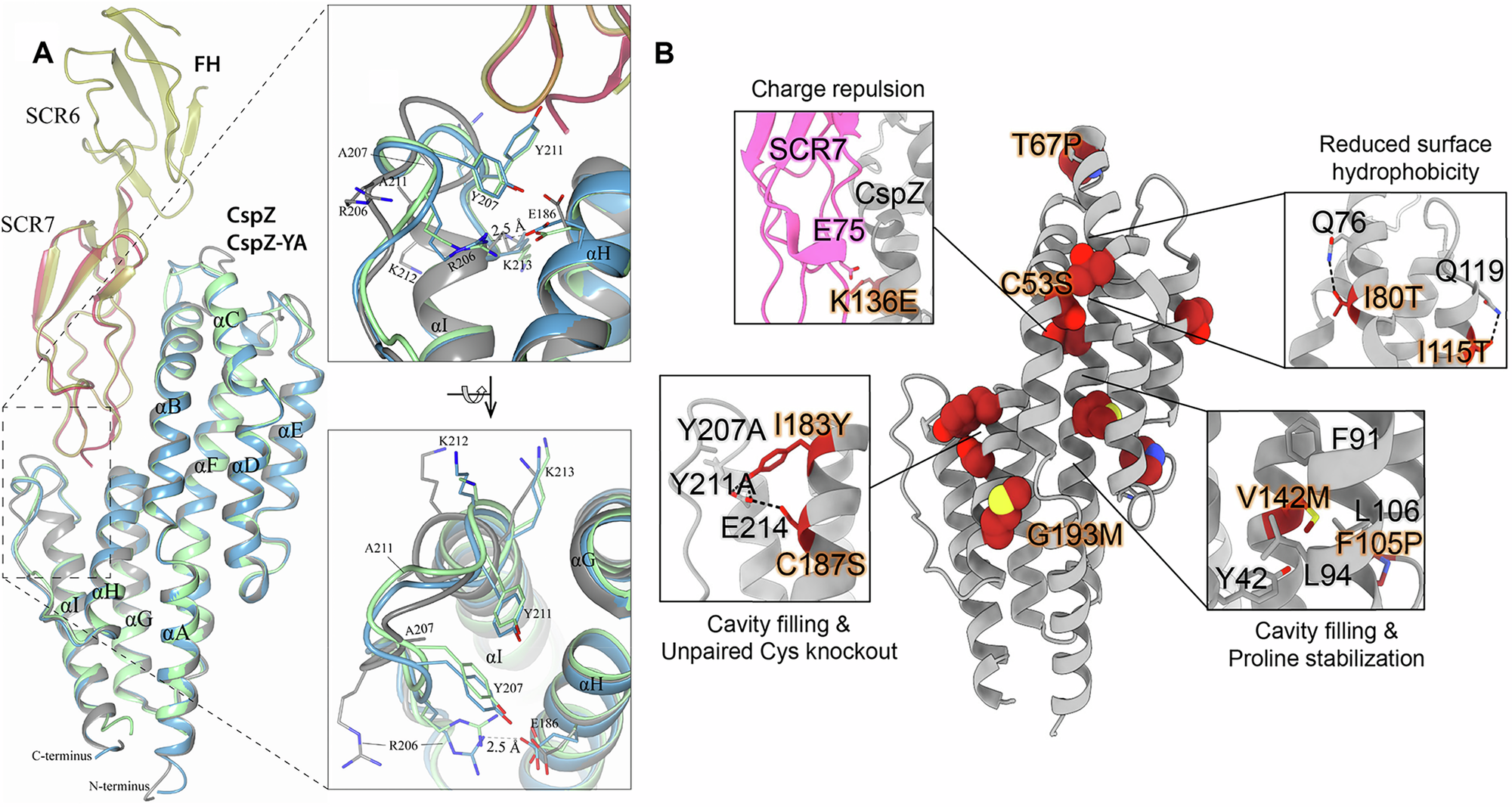
Borrelia burgdorferi (Bb) causes Lyme disease (LD), one of the most common vector-borne diseases in the Northern Hemisphere. Here, we solve the crystal structure of a mutated Bb vaccine antigen, CspZ-YA that lacks the ability to bind to host complement factor H (FH). We generate point mutants of CspZ-YA and identify CspZ-YAI183Y and CspZ-YAC187S to trigger more robust bactericidal responses. Compared to CspZ-YA, these CspZ-YA mutants require a lower immunization frequency to protect mice from LD-associated inflammation and bacterial colonization. Antigenicity of wild-type and mutant CspZ-YA proteins are similar, as measured using sera from infected people or immunized female mice. Structural comparison of CspZ-YA with CspZ-YAI183Y and CspZ-YAC187S shows enhanced interactions of two helices adjacent to the FH-binding sites in the mutants, consistent with their elevated thermostability. In line with these findings, protective CspZ-YA monoclonal antibodies show increased binding to CspZ-YA at a physiological temperature (37 °C). In summary, this proof-of-concept study applies structural vaccinology to enhance intramolecular interactions for the long-term stability of a Bb antigen while maintaining its protective epitopes, thus promoting LD vaccine development.