2025-04-17 京都大学iPS細胞研究所
<関連情報>
- https://www.cira.kyoto-u.ac.jp/j/pressrelease/news/250417-000000.html
- https://www.nature.com/articles/s41586-025-08700-0
パーキンソン病に対するiPS細胞由来ドパミン神経細胞治療の第I/II相試験 Phase I/II trial of iPS-cell-derived dopaminergic cells for Parkinson’s disease
Nobukatsu Sawamoto,Daisuke Doi,Etsuro Nakanishi,Masanori Sawamura,Takayuki Kikuchi,Hodaka Yamakado,Yosuke Taruno,Atsushi Shima,Yasutaka Fushimi,Tomohisa Okada,Tetsuhiro Kikuchi,Asuka Morizane,Satoe Hiramatsu,Takayuki Anazawa,Takero Shindo,Kentaro Ueno,Satoshi Morita,Yoshiki Arakawa,Yuji Nakamoto,Susumu Miyamoto,Ryosuke Takahashi &Jun Takahashi
Nature Published:16 April 2025
DOI:https://doi.org/10.1038/s41586-025-08700-0
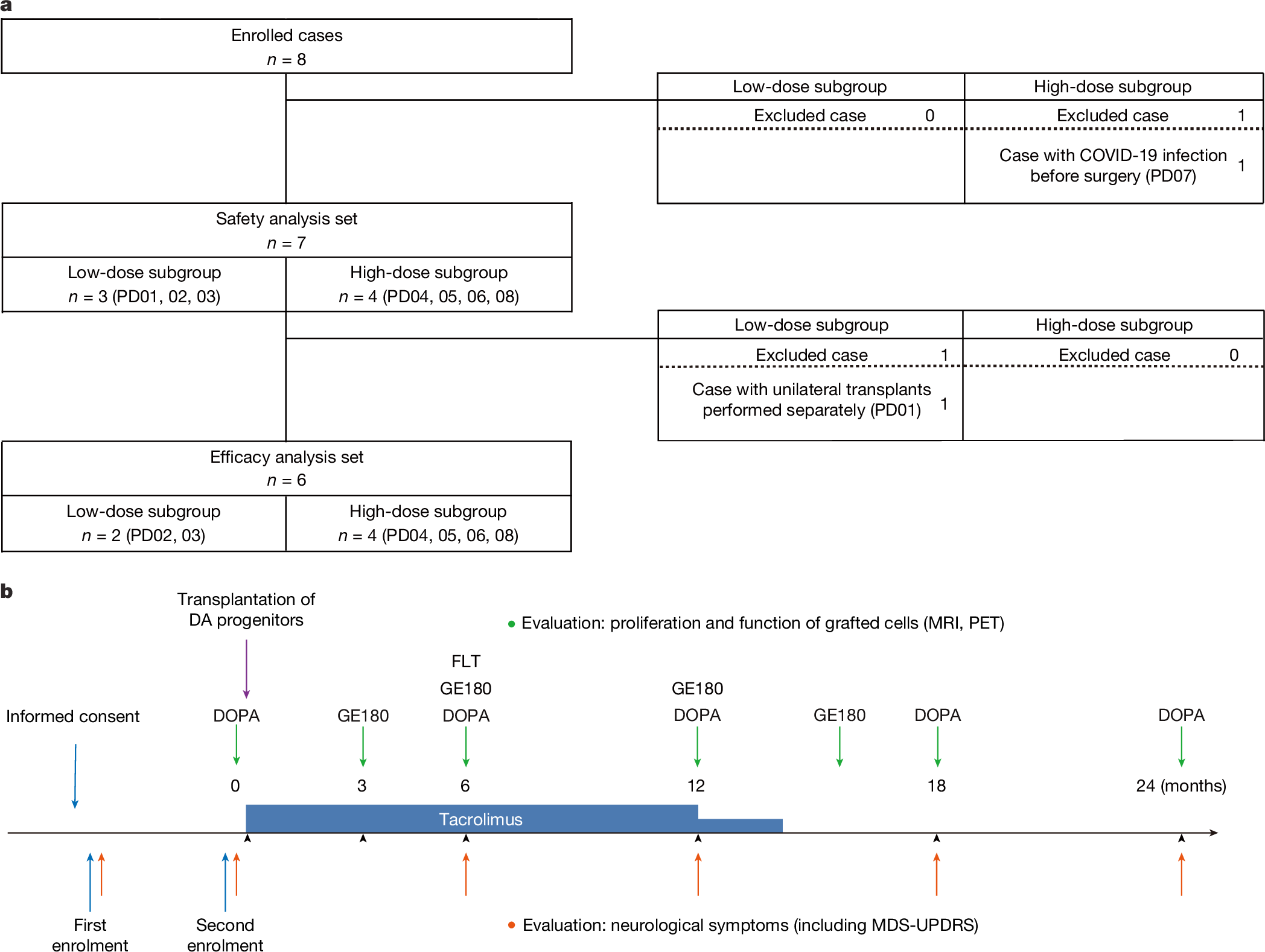
Abstract
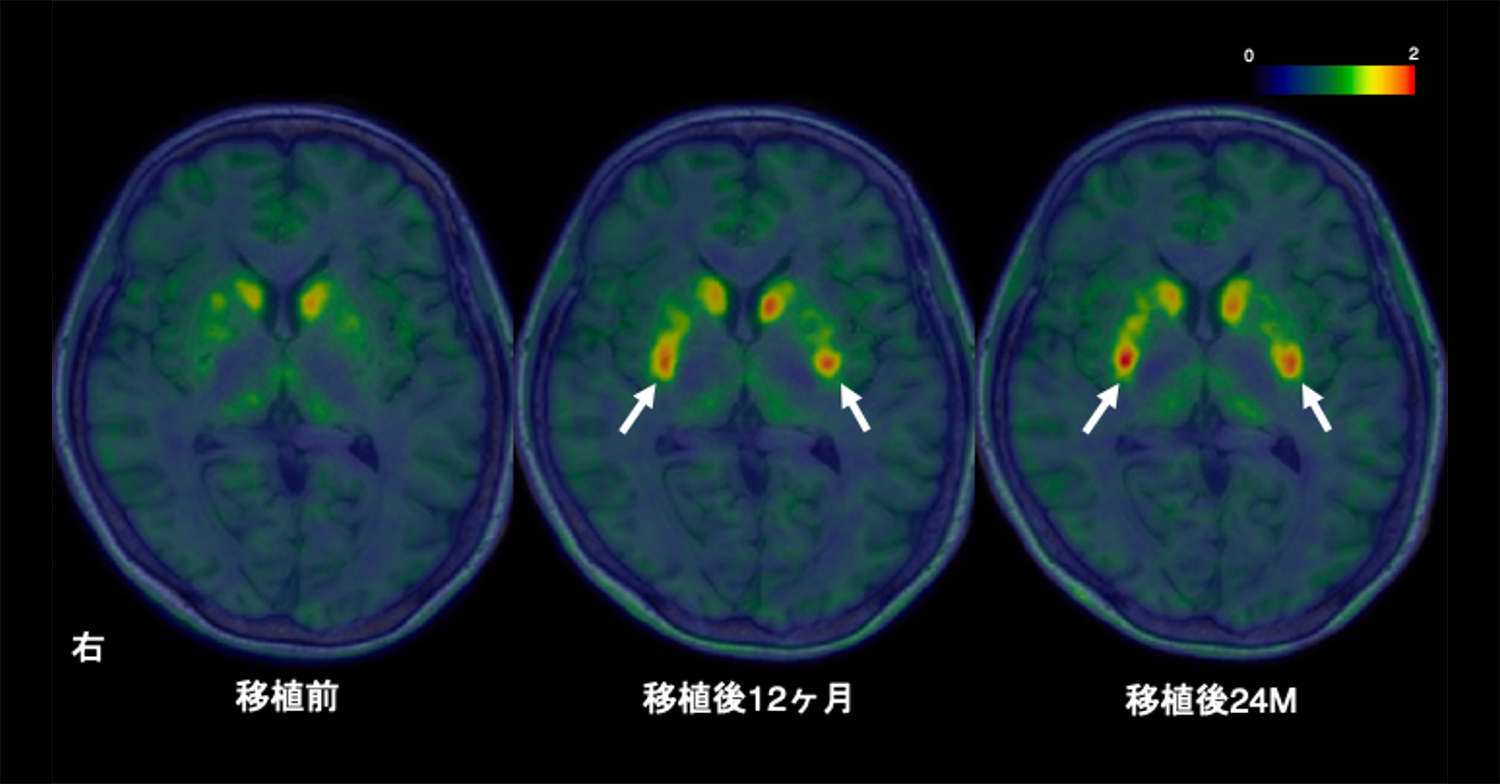
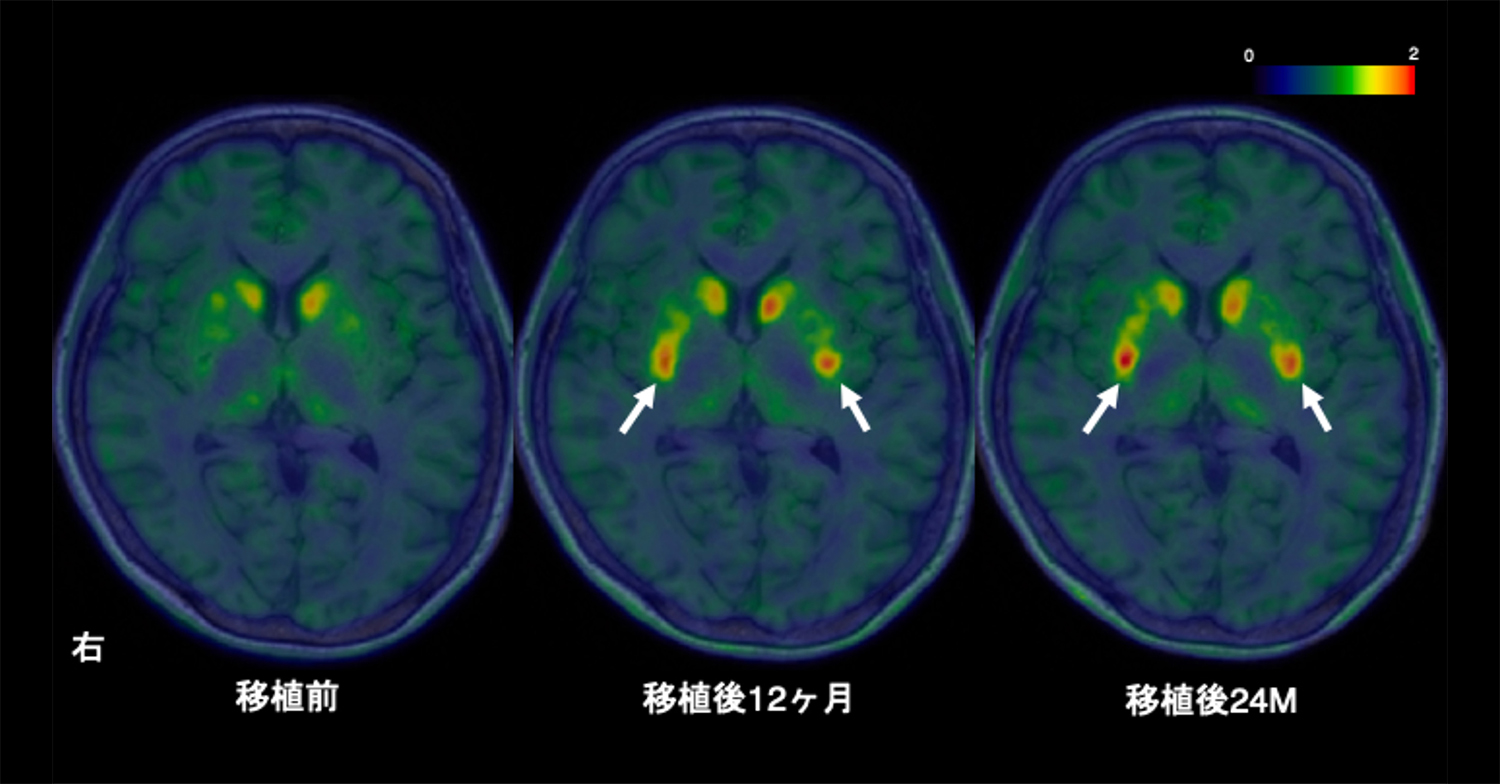
Parkinson’s disease is caused by the loss of dopamine neurons, causing motor symptoms. Initial cell therapies using fetal tissues showed promise but had complications and ethical concerns. Pluripotent stem (PS) cells emerged as a promising alternative for developing safe and effective treatments. In this phase I/II trial at Kyoto University Hospital, seven patients (ages 50–69) received bilateral transplantation of dopaminergic progenitors derived from induced PS (iPS) cells. Primary outcomes focused on safety and adverse events, while secondary outcomes assessed motor symptom changes and dopamine production for 24 months. There were no serious adverse events, with 73 mild to moderate events. Patients’ anti-parkinsonian medication doses were maintained unless therapeutic adjustments were required, resulting in increased dyskinesia. Magnetic resonance imaging showed no graft overgrowth. Among six patients subjected to efficacy evaluation, four showed improvements in the Movement Disorder Society Unified Parkinson’s Disease Rating Scale part III OFF score, and five showed improvements in the ON scores. The average changes of all six patients were 9.5 (20.4%) and 4.3 points (35.7%) for the OFF and ON scores, respectively. Hoehn–Yahr stages improved in four patients. Fluorine-18-l-dihydroxyphenylalanine (18F-DOPA) influx rate constant (Ki) values in the putamen increased by 44.7%, with higher increases in the high-dose group. Other measures showed minimal changes. This trial (jRCT2090220384) demonstrated that allogeneic iPS-cell-derived dopaminergic progenitors survived, produced dopamine and did not form tumours, therefore suggesting safety and potential clinical benefits for Parkinson’s disease.